Lyl1-deficiency promotes inflammatory responses and increases mycobacterial burden in response to Mycobacterium tuberculosis infection in mice
- PMID: 36119114
- PMCID: PMC9481033
- DOI: 10.3389/fimmu.2022.948047
Lyl1-deficiency promotes inflammatory responses and increases mycobacterial burden in response to Mycobacterium tuberculosis infection in mice
Abstract
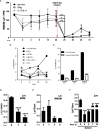
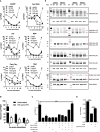
Lymphoblastic leukemia 1 (Lyl1) is a well-studied transcription factor known to exhibit oncogenic potential in various forms of leukemia with pivotal roles in hematopoietic stem cell biology. While its role in early hematopoiesis is well established, its function in mature innate cells is less explored. Here, we identified Lyl1 as a drastically perturbed gene in the Mycobacterium tuberculosis (Mtb) infected mouse macrophage transcriptome. We report that Lyl1 downregulation upon immune stimulation is a host-driven process regulated by NFκB and MAP kinase pathways. Interestingly, Lyl1-deficient macrophages have decreased bacterial killing potential with reduced nitric oxide (NO) levels while expressing increased levels of pro-inflammatory interleukin-1 and CXCL1. Lyl1-deficient mice show reduced survival to Mtb HN878 infection with increased bacterial burden and exacerbated inflammatory responses in chronic stages. We observed that increased susceptibility to infection was accompanied by increased neutrophil recruitment and IL-1, CXCL1, and CXCL5 levels in the lung homogenates. Collectively, these results suggest that Lyl1 controls Mtb growth, reduces neutrophilic inflammation and reveals an underappreciated role for Lyl1 in innate immune responses.
Keywords: Mycobacterium tuberculosis; innate immunity; lymphoblastic leukemia 1; neutrophilic inflammation; transcription factor.
Copyright © 2022 Jones, Ozturk, Kieswetter, Poswayo, Hazra, Tamgue, Parihar, Suzuki, Brombacher and Guler.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Concise review: Blood relatives: formation and regulation of hematopoietic stem cells by the basic helix-loop-helix transcription factors stem cell leukemia and lymphoblastic leukemia-derived sequence 1.Stem Cells. 2012 Jun;30(6):1053-8. doi: 10.1002/stem.1093. Stem Cells. 2012. PMID: 22593015 Review.
-
Requirement for Lyl1 in a model of Lmo2-driven early T-cell precursor ALL.Blood. 2013 Sep 19;122(12):2093-103. doi: 10.1182/blood-2012-09-458570. Epub 2013 Aug 7. Blood. 2013. PMID: 23926305
-
Overexpression of a transcription factor LYL1 induces T- and B-cell lymphoma in mice.Oncogene. 2007 Oct 18;26(48):6937-47. doi: 10.1038/sj.onc.1210494. Epub 2007 May 7. Oncogene. 2007. PMID: 17486074
-
Physical interaction of the bHLH LYL1 protein and NF-kappaB1 p105.Oncogene. 1999 Jan 28;18(4):995-1005. doi: 10.1038/sj.onc.1202374. Oncogene. 1999. PMID: 10023675
-
E Protein Transcription Factors as Suppressors of T Lymphocyte Acute Lymphoblastic Leukemia.Front Immunol. 2022 Apr 20;13:885144. doi: 10.3389/fimmu.2022.885144. eCollection 2022. Front Immunol. 2022. PMID: 35514954 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

