Adjuvant chemotherapy may improve long-term outcomes in stage IB non-small cell lung cancer patients with previous malignancies: A propensity score-matched analysis
- PMID: 36119504
- PMCID: PMC9472252
- DOI: 10.3389/fonc.2022.938195
Adjuvant chemotherapy may improve long-term outcomes in stage IB non-small cell lung cancer patients with previous malignancies: A propensity score-matched analysis
Abstract
Background: Routine administration of adjuvant chemotherapy for stage IB non-small cell lung cancer (NSCLC) remains controversial. To our knowledge, no available studies have assessed the outcomes of chemotherapy in patients with stage IB NSCLC who had prior malignancies.
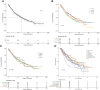
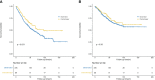
Methods: Patients with pathological stage IB NSCLC with previous malignancies who underwent surgery between 2004 and 2015 were identified from the Surveillance, Epidemiology, and End Results (SEER) database. The patients were categorized into chemotherapy and observation group based on whether they received adjuvant chemotherapy. Propensity score matching was performed to reduce confounding bias, and Kaplan-Meier curves and log-rank tests were used to compare overall survival (OS) and cancer-specific survival (CSS) between the two groups. Subgroup analyses of the matched cohorts were then conducted to evaluate the relationship between clinical features and chemotherapy.
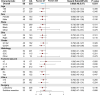
Results: A total of 894 eligible patients were identified; 90 (10.1%) patients received postoperative chemotherapy. Patients who received adjuvant chemotherapy obtained obvious OS benefits compared with those who received observation alone (HR = 0.68, 95% CI: 0.48-0.97, P = 0.031). In addition, the 5-year OS rate and median OS time in the chemotherapy group were higher and longer, respectively. Although chemotherapy offered no obvious benefits for CSS (HR = 0.80, 95% CI: 0.57-1.14, P = 0.35), patients who received chemotherapy showed a better 5-year CSS rate. On subgroup analyses, a chemotherapy advantage was observed in advanced age (≥65 years, HR = 0.62, 95% CI: 0.38-0.99, P = 0.045). The same chemotherapy advantages were observed in patients diagnosed with higher histological grades (poorly differentiated to undifferentiated) (HR = 0.56, 95% CI: 0.33-0.96, P = 0.033) and tumor sizes >3.1-4 cm (HR = 0.57, 95% CI: 0.37-0.87, P = 0.010). Interestingly, NSCLC patients with previous malignancies originating from the kidney and bladder (HR = 0.34, 95% CI: 0.12-0.99, P = 0.049) showed a chemotherapy advantage. The same chemotherapy advantages were observed in patients diagnosed with NSCLC within 3 to 5 years after prior cancers (HR = 0.39, 95% CI: 0.16-0.98, P = 0.044) and with localized SEER stage of prior cancers (HR = 0.49, 95% CI: 0.29-0.86, P = 0.012).
Conclusion: These findings indicate that adjuvant chemotherapy may improve long-term outcomes for stage IB NSCLC patients with previous malignancies. It is recommended that physicians consider the clinical features of previous cancers when making adjuvant chemotherapy decisions for these patients.
Keywords: SEER; chemotherapy; non-small cell lung cancer; previous malignancy; stage IB.
Copyright © 2022 Zhou, Zhao, Liang, Cao, Lin, Peng and Mei.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Effect of Adjuvant Chemotherapy on Survival of Patients With 8th Edition Stage IB Non-Small Cell Lung Cancer.Front Oncol. 2022 Jan 27;11:784289. doi: 10.3389/fonc.2021.784289. eCollection 2021. Front Oncol. 2022. PMID: 35155190 Free PMC article.
-
Propensity-matched analysis of adjuvant chemotherapy for completely resected Stage IB non-small-cell lung cancer patients.Lung Cancer. 2019 Jul;133:75-82. doi: 10.1016/j.lungcan.2019.04.024. Epub 2019 Apr 24. Lung Cancer. 2019. PMID: 31200832
-
Postoperative chemotherapy significantly improves survival of elderly patients with stage IB-II non-small cell lung cancer: A population-based study.Cancer Med. 2023 May;12(10):11254-11263. doi: 10.1002/cam4.5834. Epub 2023 Apr 9. Cancer Med. 2023. PMID: 37031456 Free PMC article.
-
Platinum-based adjuvant chemotherapy on moderate- and high-risk stage I and II epithelian ovarian cancer patients. Long-term single institution experience and literature review.Clin Transl Oncol. 2011 Feb;13(2):121-32. doi: 10.1007/s12094-011-0629-6. Clin Transl Oncol. 2011. PMID: 21324801 Review.
-
Role of Adjuvant Chemotherapy in Stage I Pure Ovarian Immature Teratoma: A Systematic Review and Meta-Analysis.Cancers (Basel). 2023 Mar 13;15(6):1741. doi: 10.3390/cancers15061741. Cancers (Basel). 2023. PMID: 36980627 Free PMC article. Review.
Cited by
-
Adjuvant chemotherapy in pathological node-negative non-small cell lung cancer.Sci Rep. 2023 Nov 6;13(1):19137. doi: 10.1038/s41598-023-46679-8. Sci Rep. 2023. PMID: 37932436 Free PMC article.
-
The prognostic value of visceral pleural infiltration in ≤3 cm nonsmall cell lung cancer presenting with ground glass opacity: an inverse probability of treatment weighting study.Int J Surg. 2024 Dec 1;110(12):7871-7879. doi: 10.1097/JS9.0000000000001803. Int J Surg. 2024. PMID: 38913435 Free PMC article.
-
Diagnosis, treatment, and prognosis of stage IB non-small cell lung cancer with visceral pleural invasion.Front Oncol. 2024 Jan 15;13:1310471. doi: 10.3389/fonc.2023.1310471. eCollection 2023. Front Oncol. 2024. PMID: 38288109 Free PMC article. Review.
-
A real-world retrospective study to assess efficacy and safety of alectinib as adjuvant therapy in IB-IIIB NSCLC patients harboring ALK rearrangement.Front Oncol. 2024 Oct 21;14:1422035. doi: 10.3389/fonc.2024.1422035. eCollection 2024. Front Oncol. 2024. PMID: 39497711 Free PMC article.
-
Real-world efficacy of adjuvant therapy for totally resected stage I lung adenocarcinoma patients with pathological high-risk factors: propensity score analysis.BMC Surg. 2024 May 8;24(1):140. doi: 10.1186/s12893-024-02428-w. BMC Surg. 2024. PMID: 38720305 Free PMC article.
References
-
- Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WEE, et al. . The IASLC lung cancer staging project: Proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J Thorac Oncol (2016) 11:39–51. doi: 10.1016/j.jtho.2015.09.009 - DOI - PubMed
LinkOut - more resources
Full Text Sources

