Increased sulfur-containing amino acid content and altered conformational characteristics of soybean proteins by rebalancing 11S and 7S compositions
- PMID: 36119623
- PMCID: PMC9478179
- DOI: 10.3389/fpls.2022.828153
Increased sulfur-containing amino acid content and altered conformational characteristics of soybean proteins by rebalancing 11S and 7S compositions
Abstract
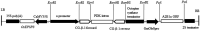
Soybean proteins are limited by their low contents of methionine and cysteine. Herein, 7S globulin accumulation was reduced using RNA interference to silence CG-β-1 expression, and the content of the A2B1a subunit was largely increased under the soybean seed-specific oleosin8 promoter. The results showed that the sulfur-containing amino acid content in soybean seeds drastically improved, reaching 79.194 nmol/mg, and the 11S/7S ratio had a 1.89-fold increase compared to the wild-type acceptor. The secondary structures of 11S globulin were also altered, and the β-sheet content increased with decreasing β-turn content, which was confirmed by Fourier transform infrared spectroscopy, Raman spectroscopy and circular dichroism analysis. Our findings suggested that raising the accumulation of 11S glycinin at the expense of reducing the content of 7S globulin is an attractive and precise engineering strategy to increase the amount of sulfur-containing amino acids, and soybean proteins with A2B1a subunits of 11S isolates improved, and β-subunits of 7S fractions reduced simultaneously might be an effective new material for food production.
Keywords: 11S glycinin; 7S globulin; RNA interference; conformational characteristics; soybean proteins; sulfur-containing amino acids.
Copyright © 2022 Wang, Teng, Yu, Yao, Ma and Wu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Rebalance between 7S and 11S globulins in soybean seeds of differing protein content and 11SA4.Food Chem. 2016 Nov 1;210:148-55. doi: 10.1016/j.foodchem.2016.04.095. Epub 2016 Apr 21. Food Chem. 2016. PMID: 27211633
-
Limited proteolysis of beta-conglycinin and glycinin, the 7S and 11S storage globulins from soybean [Glycine max (L.) Merr.]. Structural and evolutionary implications.Eur J Biochem. 1996 Oct 1;241(1):221-8. doi: 10.1111/j.1432-1033.1996.0221t.x. Eur J Biochem. 1996. PMID: 8898910
-
Quantitative Trait Loci (QTL) Mapping for Glycinin and β-Conglycinin Contents in Soybean (Glycine max L. Merr.).J Agric Food Chem. 2016 May 4;64(17):3473-83. doi: 10.1021/acs.jafc.6b00167. Epub 2016 Apr 25. J Agric Food Chem. 2016. PMID: 27070305
-
Structural and functional analysis of various globulin proteins from soy seed.Crit Rev Food Sci Nutr. 2015;55(11):1491-502. doi: 10.1080/10408398.2012.700340. Crit Rev Food Sci Nutr. 2015. PMID: 24915310 Review.
-
Pulse Globulins 11S and 7S: Origins, Purification Methods, and Techno-functional Properties.J Agric Food Chem. 2023 Feb 15;71(6):2704-2717. doi: 10.1021/acs.jafc.2c07507. Epub 2023 Feb 1. J Agric Food Chem. 2023. PMID: 36722439 Review.
Cited by
-
Effects of Germination on the Structure, Functional Properties, and In Vitro Digestibility of a Black Bean (Glycine max (L.) Merr.) Protein Isolate.Foods. 2024 Feb 3;13(3):488. doi: 10.3390/foods13030488. Foods. 2024. PMID: 38338623 Free PMC article.
-
Sulfate transport and metabolism: strategies to improve the seed protein quality.Mol Biol Rep. 2024 Feb 1;51(1):242. doi: 10.1007/s11033-023-09166-x. Mol Biol Rep. 2024. PMID: 38300326 Review.
-
Bibliometric Analysis of Functional Crops and Nutritional Quality: Identification of Gene Resources to Improve Crop Nutritional Quality through Gene Editing Technology.Nutrients. 2023 Jan 11;15(2):373. doi: 10.3390/nu15020373. Nutrients. 2023. PMID: 36678244 Free PMC article.
References
-
- Dinkins R. D., Reddy M. S. S., Meurer C. A., Yan B., Trick H., Thibaud-Nissen F., et al. . (2001). Increased sulfur amino acids in soybean plants overexpressing the maize 15 kDa zein protein. In Vitro Cell Dev. 37, 742–747. doi: 10.1007/s11627-001-0123-x - DOI
LinkOut - more resources
Full Text Sources
Research Materials

