Experiences of patients with poststroke spasticity throughout a botulinum toxin treatment cycle: Results from a prospective ethnographic study
- PMID: 36119669
- PMCID: PMC9479670
- DOI: 10.3389/fneur.2022.946500
Experiences of patients with poststroke spasticity throughout a botulinum toxin treatment cycle: Results from a prospective ethnographic study
Abstract
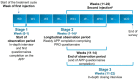
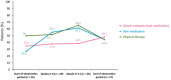
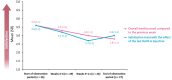
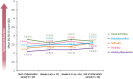
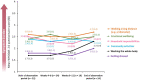
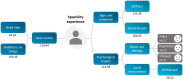
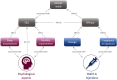
This study was conducted to capture the experience of patients with poststroke spasticity (PSS) throughout one botulinum neurotoxin A (BoNT-A) treatment cycle. The REBOT study (NCT03995524) was a prospective, observational ethnographic study conducted in France, Italy, the UK, and the USA. It combined a mixed-method ethnography (including semi-structured qualitative interviews within a week of a BoNT-A injection) with completion of a longitudinal quantitative patient-reported outcome questionnaire and sharing of video and images, both reported weekly over a 12-14-week period throughout the BoNT-A treatment cycle. The study recruited 30 adult patients with PSS who were receiving BoNT-A treatment. The most commonly used BoNT-A product was onabotulinumtoxinA (Botox®), which was administered to 21 patients (70%), whereas two patients (6.7%) received abobotulinumtoxinA (Dysport®) and seven patients (23.3%) did not specify the BoNT-A medication that they received. Patients reported a high, continuous burden of PSS, with spasms, sleeping difficulties, stiffness, and pain being the most commonly reported symptoms. In line with an observed waning effect of BoNT-A injections, spasticity symptoms initially were improved at Weeks 4-6 after injection but reemerged after 9-11 weeks. Treatment satisfaction levels decreased over the BoNT-A treatment cycle, as reflected by the worsening of symptoms and the need to self-medicate and consult a physician. The psychological impact of PSS was high. Patients acknowledged the benefits of BoNT-A treatment but wished for more individualized treatment plans with flexible dosing and injection intervals. Additionally, only 10% of patients reported that they had a trusting relationship with their physician and believed that their needs were considered by those managing their PSS. To our knowledge, this was the first ethnographic study in patients with PSS who were treated with BoNT-A. This ethnographic approach to patient surveys complements traditional research methods and allows improved identification of patients' unmet needs by capturing their weekly experience of treatment. The findings of this study confirm previous observations of the diminishing effectiveness of BoNT-A injections between treatment sessions, highlighting the need for agents with a longer duration of action and/or a more flexible treatment pattern that allows for more frequent injections.
Keywords: botulinum toxin; ethnographic study; patient-reported outcomes; poststroke spasticity; semi-structured qualitative study.
Copyright © 2022 Jacinto, Lysandropoulos, Leclerc and Calvi-Gries.
Conflict of interest statement
Author JJ received consultancy fees from Ipsen. Authors AL and FC-G are employees of Ipsen. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources

