Cordycepin enhances radiosensitivity to induce apoptosis through cell cycle arrest, caspase pathway and ER stress in MA-10 mouse Leydig tumor cells
- PMID: 36119830
- PMCID: PMC9441998
Cordycepin enhances radiosensitivity to induce apoptosis through cell cycle arrest, caspase pathway and ER stress in MA-10 mouse Leydig tumor cells
Abstract
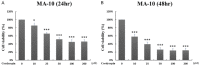
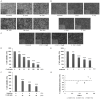
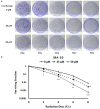
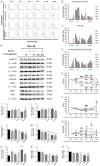
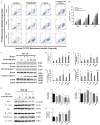
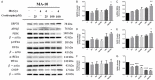
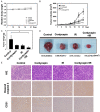
Radiotherapy is a localized treatment commonly used in various types of cancer. However, major limitation of radiotherapy is the development of resistance of tumor cells to radiosensitivity. Cordycepin, a predominant functional component of the Cordyceps sinensis, is considered to use in treating tumor cells. In the present study, we investigated the anticancer effect of the combination of radiation and cordycepin in the treatment of Leydig tumor cells. Results showed that the combination treatment has a synergistic effect significantly suppress cell viability and enhance the radiosensitivity in MA-10 mouse Leydig tumor cells. The combination treatment induced MA-10 cell apoptosis through increasing levels of cleaved caspase-3/-8/-9, poly ADP-ribose polymerase (PARP), and cytochrome c and decreasing levels of B-cell lymphoma 2 (Bcl-2). In addition, prolonged sub-G1 and G2/M arrest accompany with cell cycle-related protein regulation was observed in cells that received the combination treatment. The endoplasmic reticulum (ER) stress-related protein expressions were regulated after MA-10 cells treating with a combination of 100 μM cordycepin and 4 Gy radiation. Furthermore, the combination treatment also decreased the Leydig tumor mass by increasing cell apoptosis in tumor-bearing mice. In conclusion, cordycepin enhances radiosensitivity to induce mouse Leydig tumor cells toward apoptosis in vitro and in vivo. This study will provide a scientific basis for the development of therapeutic regimen of testicular cancer.
Keywords: Cordycepin; ER stress; Leydig tumor cell; MA-10 cell; apoptosis; caspase; cell cycle; radiation.
AJCR Copyright © 2022.
Conflict of interest statement
None.
Figures

References
-
- Richiardi L, Scelo G, Boffetta P, Hemminki K, Pukkala E, Olsen JH, Weiderpass E, Tracey E, Brewster DH, McBride ML, Kliewer EV, Tonita JM, Pompe-Kirn V, Kee-Seng C, Jonasson JG, Martos C, Brennan P. Second malignancies among survivors of germ-cell testicular cancer: a pooled analysis between 13 cancer registries. Int J Cancer. 2007;120:623–631. - PubMed
-
- Petersen PM, Skakkebaek NE, Rorth M, Giwercman A. Semen quality and reproductive hormones before and after orchiectomy in men with testicular cancer. J Urol. 1999;161:822–826. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
