Immunogenicity and safety of BNT162b2 mRNA vaccine in Chinese adults: A phase 2 randomised clinical trial
- PMID: 36120090
- PMCID: PMC9472569
- DOI: 10.1016/j.lanwpc.2022.100586
Immunogenicity and safety of BNT162b2 mRNA vaccine in Chinese adults: A phase 2 randomised clinical trial
Abstract
Background: BNT162b2, an mRNA vaccine against COVID-19, is being utilised worldwide, but immunogenicity and safety data in Chinese individuals are limited.
Methods: This phase 2, randomised, double-blind, placebo-controlled trial included healthy or medically stable individuals aged 18-85 years enrolled at two clinical sites in China. Participants were stratified by age (≤55 or >55 years) and randomly assigned (3:1) by an independent randomisation professional to receive two doses of intramuscular BNT162b2 30 μg or placebo, administered 21 days apart. Study participants, study personnel, investigators, statisticians, and the sponsor's study management team were blinded to treatment assignment. Primary immunogenicity endpoints were the geometric mean titers (GMTs) of neutralising antibodies to live severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and seroconversion rates (SCR) 1 month after the second dose. Safety assessments included reactogenicity within 14 days of vaccination, adverse events (AEs), and clinical laboratory parameters. Randomised participants who received at least one dose were included in the efficacy and safety analyses on a complete case basis (incomplete/missing data not imputed). Results up to 6 months after the second dose are reported.
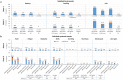
Findings: Overall, 959 participants (all of Han ethnicity) who were recruited between December 5th, 2020 and January 9th, 2021 received at least one injection (BNT162b2, n=720; placebo, n=239). At 1 month after the second dose, the 50% neutralising antibody GMT was 294.4 (95% CI; 281.1-308.4) in the BNT162b2 group and 5.0 (95% CI; 5.0-5.0) in the placebo group. SCRs were 99.7% (95% CI; 99.0%-100.0%) and 0% (95% CI; 0.0%-1.5%), respectively (p<0.0001 vs placebo). Although the GMT of neutralising antibodies in the BNT162b2 group was greatly reduced at 6 months after the second dose, the SCR still remained at 58.8%. BNT162b2-elicited sera neutralised SARS-CoV-2 variants of concern. T-cell responses were detected in 58/73 (79.5%) BNT162b2 recipients. Reactogenicity was mild or moderate in severity and resolved within a few days after onset. Unsolicited AEs were uncommon at 1 month following vaccine administration, and there were no vaccine-related serious AEs at 1 month or 6 months after the second dose.
Interpretation: BNT162b2 vaccination induced a robust immune response with acceptable tolerability in Han Chinese adults. However, follow-up duration was relatively short and COVID-19 rates were not assessed. Safety data collection is continuing until 12 months after the second dose.
Funding: BioNTech - sponsored the trial. Shanghai Fosun Pharmaceutical Development Inc. (Fosun Pharma) - conducted the trial, funded medical writing.
Clinicaltrialsgov registration number: NCT04649021. Trial status: Completed.
Keywords: BNT162b2 mRNA vaccine; COVID-19; Intramuscular injection; Messenger RNA; Neutralising antibodies; SARS-CoV-2; Vaccination.
© 2022 The Authors.
Conflict of interest statement
U.S. and Ö.T. are management board members and employees at BioNTech SE. U.S. has a leadership role at TRON Translational Oncology Mainz. He got the lecture payment from Johannes Gutenberg University as professor in 2022 and was awarded with German Future Prize 2021. Ö.T. has leadership role at HI-TRON Mainz, and is a founding member of TRON Translational Oncology Mainz. Ö.T. also got the lecture payment from Johannes Gutenberg University as professor in 2022 and was awarded with German Future Prize 2021. E.D., M.K., E.L., U.L., A.M., O.O., S.S., and Y. Shishkova are employees at BioNTech SE. M.B., S.H., and Z.K. are employees at BioNTech US. U.S., Ö.T., and A.M. are inventors on patents and patent applications related to RNA technology and COVID-19 vaccines. A.M., O.O., U.S., and Ö.T. hold securities from BioNTech SE. A.H., L.G., X.W., J.Q., W.W., and J. Zheng, are employees of Fosun Pharma. J.L., L.Z., R.T., H.Y., M.L., F.P., Z.W., X.G., Y.S., H.P., J. Zhu, Z.S., and F.Z. declare they have no conflicts of interests.
Figures

References
-
- World Health Organization. WHO Coronavirus (COVID-19) dashboard. 2022. www.covid19.who.int. Accessed 15 June 2022.
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

