Nicotine self-administration and ERK signaling are altered in RasGRF2 knockout mice
- PMID: 36120353
- PMCID: PMC9479000
- DOI: 10.3389/fphar.2022.986566
Nicotine self-administration and ERK signaling are altered in RasGRF2 knockout mice
Abstract
Ras/Raf/MEK/ERK (Ras-ERK) signaling has been demonstrated to play a role in the effects of drugs of abuse such as cocaine and alcohol, but has not been extensively examined in nicotine-related reward behaviors. We examined the role of Ras Guanine Nucleotide Releasing Factor 2 (RasGRF2), an upstream mediator of the Ras-ERK signaling pathway, on nicotine self-administration (SA) in RasGRF2 KO and WT mice. We first demonstrated that acute nicotine exposure (0.4 mg/kg) resulted in an increase in phosphorylated ERK1/2 (pERK1/2) in the striatum, consistent with previous reports. We also demonstrated that increases in pERK1/2 resulting from acute (0.4 mg/kg) and repeated (0.4 mg/kg, 10 daily injections) exposure to nicotine in WT mice were not present in RasGRF2 KO mice, confirming that RasGRF2 at least partly regulates the activity of the Ras-ERK signaling pathway following nicotine exposure. We then performed intravenous nicotine SA (0.03 mg/kg/infusion for 10 days) in RasGRF2 KO and WT mice. Consistent with a previous report using cocaine SA, RasGRF2 KO mice demonstrated an increase in nicotine SA relative to WT controls. These findings suggest a role for RasGRF2 in the reinforcing effects of nicotine, and implicate the Ras-ERK signaling pathway as a common mediator of the response to drugs of abuse.
Keywords: RasGRF2; extracellar signal-regulated kinase; nicotine; pERK; pERK1/2; self-administration (SA).
Copyright © 2022 Morella, Pohořalá, Calpe-López, Brambilla, Spanagel and Bernardi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

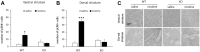
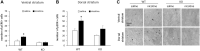

References
-
- Bertran-Gonzalez J., Bosch C., Maroteaux M., Matamales M., Herve D., Valjent E., et al. (2008). Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J. Neurosci. 28 (22), 5671–5685. 10.1523/JNEUROSCI.1039-08.2008 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

