Knockdown of RhoQ, a member of Rho GTPase, accelerates TGF-β-induced EMT in human lung adenocarcinoma
- PMID: 36120491
- PMCID: PMC9474329
- DOI: 10.1016/j.bbrep.2022.101346
Knockdown of RhoQ, a member of Rho GTPase, accelerates TGF-β-induced EMT in human lung adenocarcinoma
Abstract
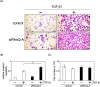
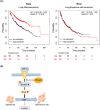
Lung cancer is the leading cause of cancer-related deaths worldwide, and the most common subtype of lung cancer is adenocarcinoma. RhoQ is a Rho family GTPase with primary sequence and structural similarities to Cdc42 and RhoJ. RhoQ is involved in neurite outgrowth via membrane trafficking and is essential for insulin-stimulated glucose uptake in mature adipocytes. However, the function of RhoQ in lung adenocarcinoma (LUAD) remains unclear. In this study, RhoQ siRNAs were introduced into A549 and PC-9 cells. Expression level of EMT-related genes and invasion ability were investigated using Western blot and transwell assay. To examine the relationship between RhoQ expression and prognosis of LUAD, Kaplan-Meier plotter was used. We discovered that suppressing RhoQ expression promoted TGF-β-mediated EMT and invasion in LUAD cell lines. Furthermore, RhoQ knockdown increased Smad3 phosphorylation and Snail expression, indicating that RhoQ was involved in TGF/Smad signaling during the EMT process. Moreover, Kaplan-Meier plotter analysis revealed that low RhoQ levels were associated with poor overall survival in patients with LUAD. In conclusion, these findings shed light on RhoQ's role as a negative regulator of TGF-β-mediated EMT in LUAD.
Keywords: Cdc42, Cell division cycle 42; DMEM, Dulbecco's Modified Eagle's Medium; EMT; EMT, Epithelial-to-Mesenchymal Transition; ERK, Extracellular signal-related kinase; FBS, Fetal Bovine Serum; LUAD, Lung adenocarcinoma; Lung adenocarcinoma; MEK, Mitogen-activated protein kinase kinase; NSCLC, Non-Small-Cell Lung Cancer; RhoQ; SDS, Sodium Dodecyl Sulfate; TGF-β; TGF-β, Transforming Growth Factor-beta; TRITC, tetramethylrhodamine-isothiocyanate; qPCR, Quantitative Polymerase Chain Reaction; rRNA, Ribosomal ribonucleic acid; siRNA, Small Interfering RNA.
© 2022 The Authors.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures


References
-
- Ramesh V., Brabletz T., Ceppi P. EMT in cancer with repurposed metabolic inhibitors. Trends in Cancer. 2020;6:942–950. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

