Afferent nerve activity in a mouse model increases with faster bladder filling rates in vitro, but voiding behavior remains unaltered in vivo
- PMID: 36121145
- PMCID: PMC9602904
- DOI: 10.1152/ajpregu.00156.2022
Afferent nerve activity in a mouse model increases with faster bladder filling rates in vitro, but voiding behavior remains unaltered in vivo
Abstract
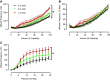
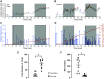
Storage and voiding functions in urinary bladder are well-known, yet fundamental physiological events coordinating these behaviors remain elusive. We sought to understand how voiding function is influenced by the rate at which the bladder fills. We hypothesized that faster filling rates would increase afferent sensory activity and increase micturition rate. In vivo, this would mean animals experiencing faster bladder filling would void more frequently with smaller void volumes. To test this hypothesis, we measured afferent nerve activity during different filling rates using an ex vivo mouse bladder preparation and assessed voiding frequency in normally behaving mice noninvasively (UroVoid). Bladder afferent nerve activity depended on the filling rate, with faster filling increasing afferent nerve activity at a given volume. Voiding behavior in vivo was measured in UroVoid cages. Male and female mice were given access to tap water or, to induce faster bladder filling rates, water containing 5% sucrose. Fluid intake increased dramatically in mice consuming 5% sucrose. As expected, micturition frequency was elevated in the sucrose group. However, even with the greatly increased rate of urine production, void volumes were unchanged in both genders. Although faster filling rates generated higher afferent nerve rates ex vivo, this did not translate into more frequent, smaller-volume voids in vivo. This suggests afferent nerve activity is only one factor contributing to the switch from bladder filling to micturition. Together with afferent nerve activity, higher centers in the central nervous system and the state of arousal are likely critical to coordinating the micturition reflex.
Keywords: incontinence; micturition; overactive bladder; polydipsia; polyuria.
Conflict of interest statement
G.M.H. is a scientific consultant at MED Associates, Inc. and Living Systems Instrumentation, a division of Catamount Research and Development, Inc., and his wife is a co-owner of these companies. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
Figures





Similar articles
-
The Effect of Bladder Filling Rate on the Voiding Behavior of Patients with Overactive Bladder.J Urol. 2019 Aug;202(2):326-332. doi: 10.1097/JU.0000000000000199. Epub 2019 Jul 8. J Urol. 2019. PMID: 30817239
-
Detrusor expulsive strength is preserved, but responsiveness to bladder filling and urinary sensitivity is diminished in the aging mouse.Am J Physiol Regul Integr Comp Physiol. 2012 Mar 1;302(5):R577-86. doi: 10.1152/ajpregu.00508.2011. Epub 2011 Dec 28. Am J Physiol Regul Integr Comp Physiol. 2012. PMID: 22204955 Free PMC article.
-
Afferent bladder nerve activity in the rat: a mechanism for starting and stopping voiding contractions.Urol Res. 2004 Dec;32(6):395-405. doi: 10.1007/s00240-004-0416-8. Epub 2004 Oct 22. Urol Res. 2004. PMID: 15517231
-
Central control of visceral pain and urinary tract function.Auton Neurosci. 2016 Oct;200:35-42. doi: 10.1016/j.autneu.2016.02.001. Epub 2016 Feb 6. Auton Neurosci. 2016. PMID: 26905459 Review.
-
Mechanisms underlying the recovery of lower urinary tract function following spinal cord injury.Prog Brain Res. 2006;152:59-84. doi: 10.1016/S0079-6123(05)52005-3. Prog Brain Res. 2006. PMID: 16198694 Review.
Cited by
-
Reflex voiding in rat occurs at consistent bladder volume regardless of pressure or infusion rate.Neurourol Urodyn. 2023 Sep;42(7):1532-1546. doi: 10.1002/nau.25243. Epub 2023 Jul 9. Neurourol Urodyn. 2023. PMID: 37583249 Free PMC article.
-
Mechanosensitive release of ATP in the urinary bladder mucosa.Purinergic Signal. 2025 Jun;21(3):413-428. doi: 10.1007/s11302-024-10063-6. Epub 2024 Nov 14. Purinergic Signal. 2025. PMID: 39541058 Free PMC article. Review.
-
Impact of surgical bladder catheter implantation on voiding function in mice.Am J Physiol Regul Integr Comp Physiol. 2025 Jun 1;328(6):R718-R726. doi: 10.1152/ajpregu.00300.2024. Epub 2025 May 7. Am J Physiol Regul Integr Comp Physiol. 2025. PMID: 40331573 Free PMC article.
-
Expression of Acid-Sensing Ion Channel 3 in Afferents Averts Long-Term Sensitization and the Development of Visceral Pain.Int J Mol Sci. 2024 Nov 21;25(23):12503. doi: 10.3390/ijms252312503. Int J Mol Sci. 2024. PMID: 39684215 Free PMC article.
-
Urothelium-derived prostanoids enhance contractility of urinary bladder smooth muscle and stimulate bladder afferent nerve activity in the mouse.Am J Physiol Regul Integr Comp Physiol. 2024 Jul 1;327(1):R97-R108. doi: 10.1152/ajpregu.00084.2024. Epub 2024 May 23. Am J Physiol Regul Integr Comp Physiol. 2024. PMID: 38780425 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

