Intestinal Norovirus Binding Patterns in Nonsecretor Individuals
- PMID: 36121297
- PMCID: PMC9555158
- DOI: 10.1128/jvi.00865-22
Intestinal Norovirus Binding Patterns in Nonsecretor Individuals
Abstract
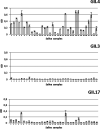
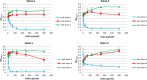
Human norovirus (HuNoV) infection is associated with an active FUT2 gene, which characterizes the secretor phenotype. However, nonsecretor individuals are also affected by HuNoV infection although in a lesser proportion. Here, we studied GII.3, GII.4, and GII.17 HuNoV interactions in nonsecretor individuals using virus-like particles (VLPs). Only GII.4 HuNoV specifically interacted with nonsecretor saliva. Competition experiments using histo-blood group antigen (HBGA)-specific monoclonal antibodies (MAbs) demonstrate that GII.4 VLPs recognized the Lewis a (Lea) antigen. We also analyzed HuNoV VLP interactions on duodenum tissue blocks from healthy nonsecretor individuals. VLP binding was observed for the three HuNoV genotypes in 10 of the 13 individuals, and competition experiments demonstrated that VLP recognition was driven by an interaction with the Lea antigen. In 3 individuals, binding was restricted to either GII.4 alone or GII.3 and GII.17. Finally, we performed a VLP binding assay on proximal and distal colon tissue blocks from a nonsecretor patient with Crohn's disease. VLP binding to inflammatory tissues was genotype specific since GII.4 and GII.17 VLPs were able to interact with regenerative mucosa, whereas GII.3 VLP was not. The binding of GII.4 and GII.17 HuNoV VLPs was linked to Lea in regenerative mucosae from the proximal and distal colon. Overall, our data clearly showed that Lea has a pivotal role in the recognition of HuNoV in nonsecretors. We also showed that Lea is expressed in inflammatory/regenerative tissues and interacts with HuNoV in a nonsecretor individual. The physiological and immunological consequences of such interactions in nonsecretors have yet to be elucidated. IMPORTANCE Human norovirus (HuNoV) is the main etiological agent of viral gastroenteritis in all age classes. HuNoV infection affects mainly secretor individuals where ABO(H) and Lewis histo-blood group antigens (HBGAs) are present in the small intestine. Nonsecretor individuals, who only express Lewis (Le) antigens, are less susceptible to HuNoV infection. Here, we studied the interaction of common HuNoV genotypes (GII.3, GII.4, and GII.17) in nonsecretor individuals using synthetic viral particles. Saliva binding assays showed that only GII.4 interacted with nonsecretor saliva via the Lewis a (Lea) antigen Surprisingly, the three genotypes interacted with nonsecretor enterocytes via the Lea antigen on duodenal tissue blocks, which were more relevant for HuNoV/HBGA studies. The Lea antigen also played a pivotal role in the recognition of GII.4 and GII.17 particles by inflammatory colon tissue from a nonsecretor Crohn's disease patient. The implications of HuNoV binding in nonsecretors remain to be elucidated in physiological and pathological conditions encountered in other intestinal diseases.
Keywords: Crohn’s disease; FUT2; HBGA; colon; duodenum; healthy; nonsecretor; norovirus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Ettayebi K, Crawford SE, Murakami K, Broughman JR, Karandikar U, Tenge VR, Neill FH, Blutt SE, Zeng XL, Qu L, Kou B, Opekun AR, Burrin D, Graham DY, Ramani S, Atmar RL, Estes MK. 2016. Replication of human noroviruses in stem cell-derived human enteroids. Science 353:1387–1393. 10.1126/science.aaf5211. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

