Trehalose-Carnosine Prevents the Effects of Spinal Cord Injury Through Regulating Acute Inflammation and Zinc(II) Ion Homeostasis
- PMID: 36121569
- PMCID: PMC10079760
- DOI: 10.1007/s10571-022-01273-w
Trehalose-Carnosine Prevents the Effects of Spinal Cord Injury Through Regulating Acute Inflammation and Zinc(II) Ion Homeostasis
Abstract
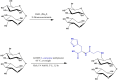
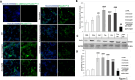
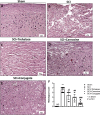
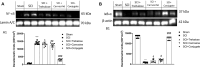
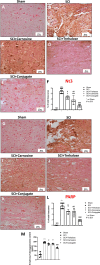
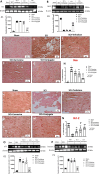
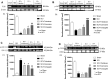
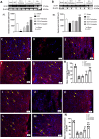
Spinal cord injury (SCI) leads to long-term and permanent motor dysfunctions, and nervous system abnormalities. Injury to the spinal cord triggers a signaling cascade that results in activation of the inflammatory cascade, apoptosis, and Zn(II) ion homeostasis. Trehalose (Tre), a nonreducing disaccharide, and L-carnosine (Car), (β-alanyl-L-histidine), one of the endogenous histidine dipeptides have been recognized to suppress early inflammatory effects, oxidative stress and to possess neuroprotective effects. We report on the effects of the conjugation of Tre with Car (Tre-car) in reducing inflammation in in vitro and in vivo models. The in vitro study was performed using rat pheochromocytoma cells (PC12 cell line). After 24 h, Tre-car, Car, Tre, and Tre + Car mixture treatments, cells were collected and used to investigate Zn2+ homeostasis. The in vivo model of SCI was induced by extradural compression of the spinal cord at the T6-T8 levels. After treatments with Tre, Car and Tre-Car conjugate 1 and 6 h after SCI, spinal cord tissue was collected for analysis. In vitro results demonstrated the ionophore effect and chelating features of L-carnosine and its conjugate. In vivo, the Tre-car conjugate treatment counteracted the activation of the early inflammatory cascade, oxidative stress and apoptosis after SCI. The Tre-car conjugate stimulated neurotrophic factors release, and influenced Zn2+ homeostasis. We demonstrated that Tre-car, Tre and Car treatments improved tissue recovery after SCI. Tre-car decreased proinflammatory, oxidative stress mediators release, upregulated neurotrophic factors and restored Zn2+ homeostasis, suggesting that Tre-car may represent a promising therapeutic agent for counteracting the consequences of SCI.
Keywords: Apoptosis; Inflammation; Ion homeostasis; Neurotrophic factors; Spinal cord injury.
© 2022. The Author(s).
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures

Similar articles
-
Ionophore Ability of Carnosine and Its Trehalose Conjugate Assists Copper Signal in Triggering Brain-Derived Neurotrophic Factor and Vascular Endothelial Growth Factor Activation In Vitro.Int J Mol Sci. 2021 Dec 16;22(24):13504. doi: 10.3390/ijms222413504. Int J Mol Sci. 2021. PMID: 34948299 Free PMC article.
-
Administration of carnosine in the treatment of acute spinal cord injury.Biochem Pharmacol. 2011 Nov 15;82(10):1478-89. doi: 10.1016/j.bcp.2011.07.074. Epub 2011 Jul 20. Biochem Pharmacol. 2011. PMID: 21787762
-
Trehalose attenuates spinal cord injury through the regulation of oxidative stress, inflammation and GFAP expression in rats.J Spinal Cord Med. 2019 May;42(3):387-394. doi: 10.1080/10790268.2018.1527077. Epub 2018 Dec 4. J Spinal Cord Med. 2019. PMID: 30513271 Free PMC article.
-
Zinc, Carnosine, and Neurodegenerative Diseases.Nutrients. 2018 Jan 29;10(2):147. doi: 10.3390/nu10020147. Nutrients. 2018. PMID: 29382141 Free PMC article. Review.
-
The Role of Interleukin-10 in the Pathogenesis and Treatment of a Spinal Cord Injury.Diagnostics (Basel). 2024 Jan 9;14(2):151. doi: 10.3390/diagnostics14020151. Diagnostics (Basel). 2024. PMID: 38248028 Free PMC article. Review.
Cited by
-
Dissociative Identity Disorder Cotreated With Zinc and L-carnosine: A Case Report.Cureus. 2024 Nov 29;16(11):e74794. doi: 10.7759/cureus.74794. eCollection 2024 Nov. Cureus. 2024. PMID: 39737293 Free PMC article.
-
Total and Free Zinc Dynamics as Biomarkers for Neurological Impairment in Traumatic Spinal Cord Injury.Nutrients. 2025 Jan 29;17(3):496. doi: 10.3390/nu17030496. Nutrients. 2025. PMID: 39940353 Free PMC article.
-
Inhibition of LRRK2 Attenuates Depression-Related Symptoms in Mice with Moderate Traumatic Brain Injury.Cells. 2023 Mar 29;12(7):1040. doi: 10.3390/cells12071040. Cells. 2023. PMID: 37048114 Free PMC article.
References
-
- Abbaszadeh F, Fakhri S, Khan H (2020) Targeting apoptosis and autophagy following spinal cord injury: therapeutic approaches to polyphenols and candidate phytochemicals. Pharmacol Res 1:1 - PubMed
-
- Abdel Baky NA, Fadda L, Al-Rasheed NM et al (2016) Neuroprotective effect of carnosine and cyclosporine—a against inflammation, apoptosis, and oxidative brain damage after closed head injury in immature rats. Toxicol Mech Methods. 10.3109/15376516.2015.1070224 - PubMed
-
- Ahsan H (2013) 3-Nitrotyrosine: a biomarker of nitrogen free radical species modified proteins in systemic autoimmunogenic conditions. Hum Immunol 1:1 - PubMed
-
- Ahshin-Majd S, Zamani S, Kiamari T et al (2016) Carnosine ameliorates cognitive deficits in streptozotocin-induced diabetic rats: possible involved mechanisms. Peptides. 10.1016/j.peptides.2016.10.008 - PubMed
-
- Aidemise Oyinbo C (2011) Secondary injury mechanisms in traumatic spinal cord injury a nugget of this multiply cascade. Acta Neurobiol Exp (wars) 70:454–467 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

