Weight Loss Outcomes Associated With Semaglutide Treatment for Patients With Overweight or Obesity
- PMID: 36121652
- PMCID: PMC9486455
- DOI: 10.1001/jamanetworkopen.2022.31982
Weight Loss Outcomes Associated With Semaglutide Treatment for Patients With Overweight or Obesity
Abstract
Importance: No retrospective cohort study has assessed the effectiveness of semaglutide at doses used in randomized clinical trials to treat obesity (ie, 1.7 and 2.4 mg).
Objective: To study weight loss outcomes associated with semaglutide treatment at doses used in randomized clinical trials for patients with overweight or obesity.
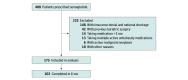
Design, setting, and participants: This cohort study, conducted at a referral center for weight management, retrospectively collected data on the use of semaglutide for adults with overweight or obesity between January 1, 2021, and March 15, 2022, with a follow-up of up to 6 months. A total of 408 patients with a body mass index (BMI) of 27 or more were prescribed weekly semaglutide subcutaneous injections for 3 months or more. Patients with a history of bariatric procedures, taking other antiobesity medications, and with an active malignant neoplasm were excluded.
Exposures: Weekly 1.7-mg or 2.4-mg semaglutide subcutaneous injections for 3 to 6 months.
Main outcomes and measures: The primary end point was the percentage of weight loss. Secondary end points were the proportion of patients achieving weight loss of 5% or more, 10% or more, 15% or more, and 20% or more after 3 and 6 months and the percentage of weight loss for patients with or without type 2 diabetes after 3 and 6 months.
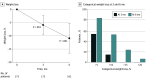
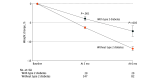
Results: The study included 175 patients (132 women [75.4%]; mean [SD] age, 49.3 [12.5] years; mean [SD] BMI, 41.3 [9.1]) in the analysis at 3 months and 102 patients at 6 months. The mean (SD) weight loss after 3 months was 6.7 (4.4) kg, equivalent to a mean (SD) weight loss of 5.9% (3.7%) (P < .001), and the mean (SD) weight loss after 6 months was 12.3 (6.6) kg, equivalent to a mean (SD) weight loss of 10.9% (5.8%) (P < .001 from baseline). Of the 102 patients who were followed up at 6 months, 89 (87.3%) achieved weight loss of 5% or more, 56 (54.9%) achieved weight loss of 10% or more, 24 (23.5%) achieved weight loss of 15% or more, and 8 (7.8%) achieved weight loss of 20% or more. Patients with type 2 diabetes had a lower mean (SD) percentage weight loss at 3 and 6 months compared with those without type 2 diabetes: 3.9% (3.1%) vs 6.3% (3.7%) at 3 months (P = .001) and 7.2% (6.3%) vs 11.8% (5.3%) at 6 months (P = .005).
Conclusions and relevance: The results of this cohort study suggest that weekly 1.7-mg and 2.4-mg doses of semaglutide were associated with weight loss similar to that seen in randomized clinical trials. Studies with longer periods of follow-up are needed to evaluate prolonged weight loss outcomes.
Conflict of interest statement
Figures
References
-
- Waters H, Graf M. America’s Obesity Crisis: The Health and Economic Costs of Excess Weight. Milken Institute; 2018.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical