Antenatal Steroid Exposure Among Term Newborns
- PMID: 36121659
- PMCID: PMC9486637
- DOI: 10.1001/jamapediatrics.2022.3251
Antenatal Steroid Exposure Among Term Newborns
Plain language summary
This cross-sectional study evaluates the association between dissemination of the Antenatal Late Preterm Steroid trial and changes in steroid exposure among term newborns.
Conflict of interest statement
Figures
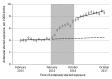
Comment in
-
The Benefit-Risk Ratio of Late Preterm Antenatal Corticosteroids Could Be Unfavorable in Real-life Situations.JAMA Pediatr. 2023 Mar 1;177(3):320-321. doi: 10.1001/jamapediatrics.2022.5255. JAMA Pediatr. 2023. PMID: 36622655 No abstract available.
-
The Benefit-Risk Ratio of Late Preterm Antenatal Corticosteroids Could Be Unfavorable in Real-life Situations-Reply.JAMA Pediatr. 2023 Mar 1;177(3):321-322. doi: 10.1001/jamapediatrics.2022.5252. JAMA Pediatr. 2023. PMID: 36622670 No abstract available.
References
-
- Clapp MA, Melamed A, Freret TS, James KE, Gyamfi-Bannerman C, Kaimal AJ. US incidence of late-preterm steroid use and associated neonatal respiratory morbidity after publication of the Antenatal Late Preterm Steroid trial, 2015-2017. JAMA Netw Open. 2022;5(5):e2212702. doi:10.1001/jamanetworkopen.2022.12702 - DOI - PMC - PubMed
-
- Reddy UM, Deshmukh U, Dude A, Harper L, Osmundson SS; Society for Maternal-Fetal Medicine (SMFM) . Society for Maternal-Fetal Medicine consult series #58: use of antenatal corticosteroids for individuals at risk for late preterm delivery: replaces SMFM statement #4, implementation of the use of antenatal corticosteroids in the late preterm birth period in women at risk for preterm delivery, August 2016. Am J Obstet Gynecol. 2021;225(5):B36-B42. doi:10.1016/j.ajog.2021.07.023 - DOI - PubMed

