Magnetoencephalography contrast adaptation reflects perceptual adaptation
- PMID: 36121660
- PMCID: PMC9503227
- DOI: 10.1167/jov.22.10.16
Magnetoencephalography contrast adaptation reflects perceptual adaptation
Abstract
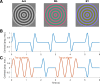
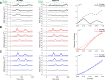
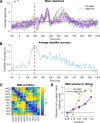
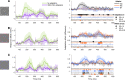
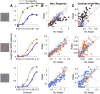
Contrast adaptation is a fundamental visual process that has been extensively investigated and used to infer the selectivity of visual cortex. We recently reported an apparent disconnect between the effects of contrast adaptation on perception and functional magnetic resonance imaging BOLD response adaptation, in which adaptation between chromatic and achromatic stimuli measured psychophysically showed greater selectivity than adaptation measured using BOLD signals. Here we used magnetoencephalography (MEG) recordings of neural responses to the same chromatic and achromatic adaptation conditions to characterize the neural effects of contrast adaptation and to determine whether BOLD adaptation or MEG better reflect the measured perceptual effects. Participants viewed achromatic, L-M isolating, or S-cone isolating radial sinusoids before adaptation and after adaptation to each of the three contrast directions. We measured adaptation-related changes in the neural response to a range of stimulus contrast amplitudes using two measures of the MEG response: the overall response amplitude, and a novel time-resolved measure of the contrast response function, derived from a classification analysis combined with multidimensional scaling. Within-stimulus adaptation effects on the contrast response functions in each case showed a pattern of contrast-gain or a combination of contrast-gain and response-gain effects. Cross-stimulus adaptation conditions showed that adaptation effects were highly stimulus selective across early, ventral, and dorsal visual cortical areas, consistent with the perceptual effects.
Figures



References
-
- Bartsch, M. V., Loewe, K., Merkel, C., Heinze, H.-J., Schoenfeld, M. A., Tsotsos, J. K., & Hopf, J.-M. (2017). Attention to Color Sharpens Neural Population Tuning via Feedback Processing in the Human Visual Cortex Hierarchy. Journal of Neuroscience, 37(43), 10346–10357, 10.1523/JNEUROSCI.0666-17.2017. - DOI - PMC - PubMed
-
- Benjamini, Y., & Hochberg, Y. (1995). Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. Series B (Methodological), 57(1), 289–300.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

