External drivers of BOLD signal's non-stationarity
- PMID: 36121808
- PMCID: PMC9484685
- DOI: 10.1371/journal.pone.0257580
External drivers of BOLD signal's non-stationarity
Abstract
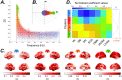
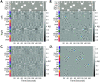
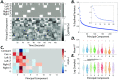
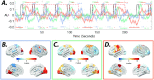
A fundamental challenge in neuroscience is to uncover the principles governing how the brain interacts with the external environment. However, assumptions about external stimuli fundamentally constrain current computational models. We show in silico that unknown external stimulation can produce error in the estimated linear time-invariant dynamical system. To address these limitations, we propose an approach to retrieve the external (unknown) input parameters and demonstrate that the estimated system parameters during external input quiescence uncover spatiotemporal profiles of external inputs over external stimulation periods more accurately. Finally, we unveil the expected (and unexpected) sensory and task-related extra-cortical input profiles using functional magnetic resonance imaging data acquired from 96 subjects (Human Connectome Project) during the resting-state and task scans. This dynamical systems model of the brain offers information on the structure and dimensionality of the BOLD signal's external drivers and shines a light on the likely external sources contributing to the BOLD signal's non-stationarity. Our findings show the role of exogenous inputs in the BOLD dynamics and highlight the importance of accounting for external inputs to unravel the brain's time-varying functional dynamics.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

