Stereoselective Synthesis of Bisfuranoxide (Aurochrome, Auroxanthin) and Monofuranoxide (Equinenone 5',8'-Epoxide) Carotenoids by Double Horner-Wadsworth-Emmons Reaction
- PMID: 36121920
- PMCID: PMC9693700
- DOI: 10.1021/acs.jnatprod.2c00475
Stereoselective Synthesis of Bisfuranoxide (Aurochrome, Auroxanthin) and Monofuranoxide (Equinenone 5',8'-Epoxide) Carotenoids by Double Horner-Wadsworth-Emmons Reaction
Abstract
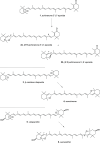
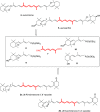
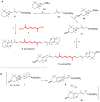
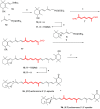
The stereoselective synthesis of C40-all-trans-carotenoids with the formal hexahydrobenzofuran skeletons aurochrome, auroxanthin, and equinenone-5',8'-epoxide is reported. The synthesis is based on a one-pot or stepwise double Horner-Wadsworth-Emmons (HWE) reaction of a terminal enantiopure C15-5,6-epoxycyclohexadienylphosphonate and a central C10-trienedial. The ring expansion of the epoxycyclohexadienylphosphonate, generated by a Stille cross-coupling reaction, to the hexahydrobenzofuran skeleton was promoted by the reaction conditions of the HWE reaction prior to double-bond formation.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Rodriguez-Concepcion M.; Avalos J.; Bonet M. L.; Boronat A.; Gomez-Gomez L.; Hornero-Mendez D.; Limon M. C.; Meléndez-Martínez A. J.; Olmedilla-Alonso B.; Palou A.; Ribot J.; Rodrigo M. J.; Zacarias L.; Zhu C. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid Res. 2018, 70, 62–93. 10.1016/j.plipres.2018.04.004. - DOI - PubMed
-
- Britton G.; Liaaen-Jensen S.; Pfander H. E.. Carotenoids. Part 1A. Isolation and Analysis. Birkhäuser: Basel, 1995.
-
- Britton G.; Liaaen-Jensen S.; Pfander H.; Eds.; Carotenoids. Part 1B. Spectroscopy; Birkhäuser: Basel, 1995.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

