Tobacco smoke exacerbates Filifactor alocis pathogenicity
- PMID: 36122937
- PMCID: PMC9976951
- DOI: 10.1111/jcpe.13729
Tobacco smoke exacerbates Filifactor alocis pathogenicity
Abstract
Aim: Filifactor alocis has recently emerged as a periodontal pathobiont that appears to thrive in the oral cavity of smokers. We hypothesized that identification of smoke-responsive F. alocis genes would provide insight into adaptive strategies and that cigarette smoke would enhance F. alocis pathogenesis in vivo.
Materials and methods: F. alocis was grown in vitro and cigarette smoke extract-responsive genes determined by RNAseq. Mice were exposed, or not, to mainstream 1R6F research cigarette smoke and infected with F. alocis, or not, in an acute ligature model of periodontitis. Key clinical, infectious, and immune data were collected.
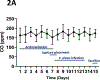
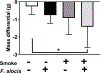
Results: In culture, F. alocis growth was unaffected by smoke conditioning and only a small number of genes were specifically regulated by smoke exposure. Reduced murine mass, differences in F. alocis-cognizant antibody production, and altered immune profiles as well as altered alveolar bone loss were all attributable to smoke exposure and/or F. alocis infection in vivo.
Conclusions: F. alocis is well-adapted to tobacco-rich conditions and its pathogenesis is enhanced by tobacco smoke exposure. A smoke-exposed ligature model of periodontitis shows promise as a tool with which to further unravel mechanisms underlying tobacco-enhanced, bacteria-induced disease.
Keywords: Filifactor alocis; alveolar bone loss; experimental periodontitis; microbiology; tobacco smoking.
© 2022 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
Figures




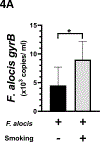
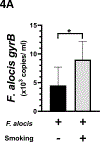
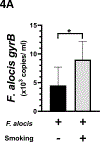

References
-
- Perez-Chaparro PJ, et al. , Do different probing depths exhibit striking differences in microbial profiles? J Clin Periodontol, 2018. 45(1): p. 26–37. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

