MRI techniques for immunotherapy monitoring
- PMID: 36122963
- PMCID: PMC9486399
- DOI: 10.1136/jitc-2022-004708
MRI techniques for immunotherapy monitoring
Erratum in
-
Correction: MRI techniques for immunotherapy monitoring.J Immunother Cancer. 2022 Nov;10(11):e004708corr1. doi: 10.1136/jitc-2022-004708corr1. J Immunother Cancer. 2022. PMID: 36328379 Free PMC article. No abstract available.
Abstract
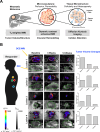
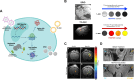
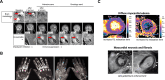
MRI is a widely available clinical tool for cancer diagnosis and treatment monitoring. MRI provides excellent soft tissue imaging, using a wide range of contrast mechanisms, and can non-invasively detect tissue metabolites. These approaches can be used to distinguish cancer from normal tissues, to stratify tumor aggressiveness, and to identify changes within both the tumor and its microenvironment in response to therapy. In this review, the role of MRI in immunotherapy monitoring will be discussed and how it could be utilized in the future to address some of the unique clinical questions that arise from immunotherapy. For example, MRI could play a role in identifying pseudoprogression, mixed response, T cell infiltration, cell tracking, and some of the characteristic immune-related adverse events associated with these agents. The factors to be considered when developing MRI imaging biomarkers for immunotherapy will be reviewed. Finally, the advantages and limitations of each approach will be discussed, as well as the challenges for future clinical translation into routine clinical care. Given the increasing use of immunotherapy in a wide range of cancers and the ability of MRI to detect the microstructural and functional changes associated with successful response to immunotherapy, the technique has great potential for more widespread and routine use in the future for these applications.
Keywords: Immunologic Techniques; Immunotherapy; Tumor Biomarkers.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: The authors declare the following competing interests: DL has research support from AstraZeneca. PGC has received speaker fees and Advisory Board Honoraria from Bristol Myers Squibb, Merck Sharp & Dohme, Novartis and Pierre Fabre. FAG has research support from GE Healthcare, grants from GlaxoSmithKline, and has consulted for AstraZeneca on behalf of the University of Cambridge.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
