Structural Diversity within the Endoplasmic Reticulum-From the Microscale to the Nanoscale
- PMID: 36123032
- PMCID: PMC10394098
- DOI: 10.1101/cshperspect.a041259
Structural Diversity within the Endoplasmic Reticulum-From the Microscale to the Nanoscale
Abstract
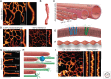
The endoplasmic reticulum (ER) is a continuous, highly dynamic membrane compartment that is crucial for numerous basic cellular functions. The ER stretches from the nuclear envelope to the outer periphery of all living eukaryotic cells. This ubiquitous organelle shows remarkable structural complexity, adopting a range of shapes, curvatures, and length scales. Canonically, the ER is thought to be composed of two simple membrane elements: sheets and tubules. However, recent advances in superresolution light microscopy and three-dimensional electron microscopy have revealed an astounding diversity of nanoscale ER structures, greatly expanding our view of ER organization. In this review, we describe these diverse ER structures, focusing on what is known of their regulation and associated functions in mammalian cells.
Copyright © 2023 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures





References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
