The Role of Myofibroblasts in Physiological and Pathological Tissue Repair
- PMID: 36123034
- PMCID: PMC9808581
- DOI: 10.1101/cshperspect.a041231
The Role of Myofibroblasts in Physiological and Pathological Tissue Repair
Abstract
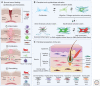
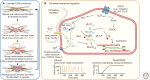
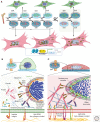
Myofibroblasts are the construction workers of wound healing and repair damaged tissues by producing and organizing collagen/extracellular matrix (ECM) into scar tissue. Scar tissue effectively and quickly restores the mechanical integrity of lost tissue architecture but comes at the price of lost tissue functionality. Fibrotic diseases caused by excessive or persistent myofibroblast activity can lead to organ failure. This review defines myofibroblast terminology, phenotypic characteristics, and functions. We will focus on the central role of the cell, ECM, and tissue mechanics in regulating tissue repair by controlling myofibroblast action. Additionally, we will discuss how therapies based on mechanical intervention potentially ameliorate wound healing outcomes. Although myofibroblast physiology and pathology affect all organs, we will emphasize cutaneous wound healing and hypertrophic scarring as paradigms for normal tissue repair versus fibrosis. A central message of this review is that myofibroblasts can be activated from multiple cell sources, varying with local environment and type of injury, to either restore tissue integrity and organ function or create an inappropriate mechanical environment.
Copyright © 2023 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
