Profiling the real-world management status of high-risk human papillomavirus infection: a protocol to establish a prospective cohort of high-risk human papillomavirus-infected women in Lueyang County, China
- PMID: 36123075
- PMCID: PMC9486292
- DOI: 10.1136/bmjopen-2022-062678
Profiling the real-world management status of high-risk human papillomavirus infection: a protocol to establish a prospective cohort of high-risk human papillomavirus-infected women in Lueyang County, China
Abstract
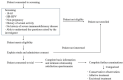
Introduction: Persistent infection with high-risk human papillomavirus (hrHPV) is the main cause of cervical cancer. Thus, the effective treatment against HPV represents an opportunity to reduce the incidence of cervical cancer. Although various treatments are effective in treating HPV infection, they still provide limited benefit in reducing the rate of cervical cancer due to the lack of implementation of a standardised protocol in many low/middle-income areas. This proposed cohort study aims to describe the status quo of treatment, attributions of the treatment decision-making process and potential factors influencing treatment decisions.
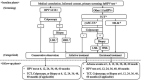
Methods and analysis: This is a mixed-method, 5-year prospective longitudinal study in Lueyang County, China, one of the areas with the highest cervical cancer incidence rates and lowest mean income in China. We will enrol women with hrHPV infection (at least one HPV type in the 13 high-risk subtypes) diagnosed via a county-wide HPV infection and cervical cancer screening programme. The study procedures describe the treatment patterns and explore the potential influencing factors in treatment decision-making through questionnaires, laboratory examinations and in-depth interviews. All participants will be evaluated at baseline and at 6, 12, 24, 36, 48 and 60 months. The primary outcome is the treatment pattern, the type and duration of which will be described later. The secondary outcomes include guideline compliance and changes in the HPV infection status. The HPV impact profile, intimate relationship satisfaction, and costs within different management groups are also described and compared.
Ethics and dissemination: This study was reviewed, and all of the relevant approvals were obtained from the Ethics Committee of the Maternity Service Centre of Lueyang Maternal and Child Health Care Hospital (2021-001). The findings from this study will be disseminated through peer-reviewed publications, conference presentations and academic workshops.
Trial registration number: ChiCTR2100053757.
Keywords: EPIDEMIOLOGY; Infection control; Public health.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Real-world effectiveness of primary screening with high-risk human papillomavirus testing in the cervical cancer screening programme in China: a nationwide, population-based study.BMC Med. 2021 Jul 15;19(1):164. doi: 10.1186/s12916-021-02026-0. BMC Med. 2021. PMID: 34261463 Free PMC article.
-
Study protocol of the ACCESS trial: a randomised trial to evaluate the effectiveness of human papillomavirus testing by self-sampling in cervical cancer screening uptake and precancer detection.BMJ Open. 2022 Feb 3;12(2):e049803. doi: 10.1136/bmjopen-2021-049803. BMJ Open. 2022. PMID: 35115348 Free PMC article.
-
Changes in High-Risk HPV Infection Prevalence and Associated Factors in Selected Rural Areas of China: A Multicenter Population-Based Study.Front Med (Lausanne). 2022 Jul 12;9:911367. doi: 10.3389/fmed.2022.911367. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35903319 Free PMC article.
-
Review of the cervical cancer disease burden in mainland China.Asian Pac J Cancer Prev. 2011;12(5):1149-53. Asian Pac J Cancer Prev. 2011. PMID: 21875257 Review.
-
Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer.Vaccine. 2012 Nov 20;30 Suppl 5:F88-99. doi: 10.1016/j.vaccine.2012.06.095. Vaccine. 2012. PMID: 23199969 Review.
Cited by
-
Knowledge and Practices Regarding Human Papillomavirus and Cervical Cancer Screening Among Women in Low-Income Areas of China: A Cross-Sectional Study.Cureus. 2024 Mar 11;16(3):e55930. doi: 10.7759/cureus.55930. eCollection 2024 Mar. Cureus. 2024. PMID: 38601389 Free PMC article.
-
Prevalence of High-Risk Human Papillomavirus Infection, Associated Risk Factors, and Relationship With Cervical Precancerous Lesions in Perimenopausal and Older Women in an Area With High Cervical Cancer Incidence in China.Cureus. 2024 Apr 11;16(4):e58081. doi: 10.7759/cureus.58081. eCollection 2024 Apr. Cureus. 2024. PMID: 38616979 Free PMC article.
-
Women's self-reported symptoms of reproductive tract infection, medical consultations, and factors influencing them in less developed regions: perimenopausal and older women in need of urgent attention.Front Public Health. 2024 Oct 22;12:1401474. doi: 10.3389/fpubh.2024.1401474. eCollection 2024. Front Public Health. 2024. PMID: 39502817 Free PMC article.
References
-
- Jie H. China cancer registration annual report 2018[M. 6163. Beijing: People’s Publishing House, 2019.
