A functional selection reveals previously undetected anti-phage defence systems in the E. coli pangenome
- PMID: 36123438
- PMCID: PMC9519451
- DOI: 10.1038/s41564-022-01219-4
A functional selection reveals previously undetected anti-phage defence systems in the E. coli pangenome
Erratum in
-
Author Correction: A functional selection reveals previously undetected anti-phage defence systems in the E. coli pangenome.Nat Microbiol. 2024 Oct;9(10):2760-2761. doi: 10.1038/s41564-024-01724-8. Nat Microbiol. 2024. PMID: 38769436 Free PMC article. No abstract available.
Abstract
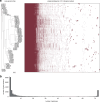
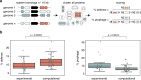
The ancient, ongoing coevolutionary battle between bacteria and their viruses, bacteriophages, has given rise to sophisticated immune systems including restriction-modification and CRISPR-Cas. Many additional anti-phage systems have been identified using computational approaches based on genomic co-location within defence islands, but these screens may not be exhaustive. Here we developed an experimental selection scheme agnostic to genomic context to identify defence systems in 71 diverse E. coli strains. Our results unveil 21 conserved defence systems, none of which were previously detected as enriched in defence islands. Additionally, our work indicates that intact prophages and mobile genetic elements are primary reservoirs and distributors of defence systems in E. coli, with defence systems typically carried in specific locations or hotspots. These hotspots encode dozens of additional uncharacterized defence system candidates. Our findings reveal an extended landscape of antiviral immunity in E. coli and provide an approach for mapping defence systems in other species.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures









References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

