Transcription factor AP2 enhances malignancy of non-small cell lung cancer through upregulation of USP22 gene expression
- PMID: 36123698
- PMCID: PMC9484186
- DOI: 10.1186/s12964-022-00946-9
Transcription factor AP2 enhances malignancy of non-small cell lung cancer through upregulation of USP22 gene expression
Abstract
Background: Ubiquitin-specific protease 22 (USP22), a putative cancer stem cell marker, is frequently upregulated in cancers, and USP22 overexpression is associated with aggressive growth, metastasis, and therapy resistance in various human cancers including lung cancer. However, USP22 gene amplification seldom occurs, and the mechanism underlying USP22 upregulation in human cancers remains largely unknown.
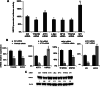
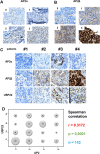
Methods: A luciferase reporter driven by a promoter region of USP22 gene was selectively constructed to screen against a customized siRNA library targeting 89 selected transcription factors to identify potential transcription factors (TFs) that regulate USP22 expression in human non-small cell lung cancers (NSCLC). Association of identified TFs with USP22 and potential role of the TFs were validated and explored in NSCLC by biological assays and immunohistochemistry analysis.
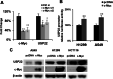
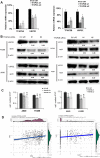
Results: Luciferase reporter assays revealed that SP1 and activating transcription factor 3 (ATF3) inhibit USP22 transcription, while transcription factor AP-2 Alpha/Beta (TFAP2A/2B) and c-Myc promote USP22 transcription. Binding site-directed mutagenesis and chromosome immunoprecipitation (ChIP) assays validated AP2α and AP2β are novel TFs of USP22. Furthermore, overexpression of AP2A and AP2B significantly upregulates USP22 expression, and its target: Cyclin D1, concurrently enhances the proliferation, migration, and invasion of NSCLC A549 and H1299 cells in a partially USP22-dependent manner. Moreover, AP2 protein level correlated with USP22 protein in human NSCLC tissues.
Conclusion: Our findings indicate AP2α and AP2β are important transcription factors driving USP22 gene expression to promote the progression of NSCLC, and further support USP22 as a potential biomarker and therapeutic target for lung cancer. Video Abstract.
Keywords: AP2; ATF3; Cell proliferation; Invasion; Malignancy; NSCLC; Transcriptional regulation; USP22.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Zhang XY, Varthi M, Sykes SM, Phillips C, Warzecha C, Zhu W, Wyce A, Thorne AW, Berger SL, McMahon SB. The putative cancer stem cell marker USP22 is a subunit of the human SAGA complex required for activated transcription and cell-cycle progression. Mol Cell. 2008;29(1):102–111. doi: 10.1016/j.molcel.2007.12.015. - DOI - PMC - PubMed
-
- Gennaro VJ, Stanek TJ, Peck AR, Sun Y, Wang F, Qie S, Knudsen KE, Rui H, Butt T, Diehl JA, et al. Control of CCND1 ubiquitylation by the catalytic SAGA subunit USP22 is essential for cell cycle progression through G1 in cancer cells. Proc Natl Acad Sci USA. 2018;115(40):E9298–E9307. doi: 10.1073/pnas.1807704115. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

