Ex vivo susceptibility-weighted imaging anatomy of canine brain-comparison of imaging and histological sections
- PMID: 36124091
- PMCID: PMC9481421
- DOI: 10.3389/fnana.2022.948159
Ex vivo susceptibility-weighted imaging anatomy of canine brain-comparison of imaging and histological sections
Abstract
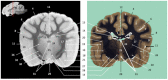
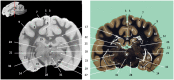
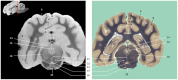
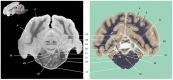
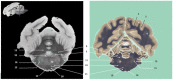
Now that access of large domestic mammals to high-field MRI becomes more common, techniques initially implemented for human patients can be used for the structural and functional study of the brain of these animals. Among them, susceptibility-weighted imaging (SWI) is a recent technique obtained from gradient echo (GE) imaging that allow for an excellent anatomical tissue contrast and a non-invasive assessment of brain iron content. The goal of this study was to design an optimal GE SWI imaging protocol to be used in dogs undergoing an MRI examination of the brain in a 3-Tesla scanner. This imaging protocol was applied to ex vivo brains from four dogs. The imaging protocol was validated by visual inspection of the SWI images that provided a high anatomical detail, as demonstrated by their comparison with corresponding microscopic sections. As resolvable brain structures were labeled, this study is the first to provide an anatomic description of SWI images of the canine brain. Once validated in living animals, this GE SWI imaging protocol could be easily included in routine neuroimaging protocols to improve the diagnosis of various intracranial diseases of dogs, or be used in future comparative studies aiming at evaluating brain iron content in animals.
Keywords: atlas; brain; canine; histology; susceptibility-weighted imaging.
Copyright © 2022 Arribarat, Cartiaux, Boucher, Montel, Gros-Dagnac, Fave, Péran, Mogicato and Deviers.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Carrera I., Richter H., Beckmann K., Meier D., Dennler M., Kircher P. R. (2016). Evaluation of intracranial neoplasia and noninfectious meningoencephalitis in dogs by use of short echo time, single voxel proton magnetic resonance spectroscopy at 3.0 Tesla. Am. J. Vet. Res. 77, 452–462. 10.2460/ajvr.77.5.452 - DOI - PubMed
LinkOut - more resources
Full Text Sources

