Rapid and Simultaneous Quantification of Six Aristolochic Acids and Two Lignans in Asari Radix et Rhizoma Using Ultra-Performance Liquid Chromatography-Triple Quadrupole Tandem Mass Spectrometry
- PMID: 36124165
- PMCID: PMC9482547
- DOI: 10.1155/2022/5269545
Rapid and Simultaneous Quantification of Six Aristolochic Acids and Two Lignans in Asari Radix et Rhizoma Using Ultra-Performance Liquid Chromatography-Triple Quadrupole Tandem Mass Spectrometry
Abstract
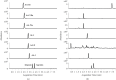
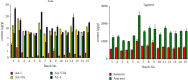
Asari Radix et Rhizoma (AR) is a widely-used Chinese herbal medicine containing multiple active lignans and rare nephrotoxic components-aristolochic acids derivatives (AAs). However, the current quality control method carried out by Chinese Pharmacopoeia has defects in trace AAs detection and insufficient marker ingredients, which is unable to comprehensively evaluate the efficacy and safety of AR. To improve the quality control method of AR, a rapid, sensitive, and reliable chromatographic analytic method based on ultra-high-performance liquid chromatography-triple quadrupole tandem mass spectrometry (UHPLC-QqQ-MS) was established for the simultaneous analysis of multiple AAs and lignans in AR samples. Positive electrospray ionization mode with multiple reaction monitoring (MRM) was applied for the detection of the eight analytes. The method showed available linearity (R 2 ≥ 0.991), the limit of quantification (2-5 ng/mL), precision (RSD <8.12%), and accuracy (89.78-112.16%). A total of 6 AAs and 2 lignans were quantified for their content in 15 AR samples. The content of AA-IVa, AA-VIIa, and aristololactam I (AL-I) was much higher than the AA-I controlled by pharmacopoeia. Considering the potential toxicity of AAs, AA-IVa, AA-VIIa, and AL-I should also be controlled in AR. A considerable amount of active sesamin was detected in AR, suggesting that it could be added as a quality marker for the quality control of AR. The newly developed analytical method could be applied for the fast evaluation of toxic AA's content and quality during quality control of AR or preparations containing AR.
Copyright © 2022 Hanze Liu et al.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures
References
-
- Committee of National Pharmacopoeia. Pharmacopoeia of People’s Republic of China . Beijing, China: China Medical Science and Technology Press; 2020.
-
- Kim E. J., Chen Y., Huang J. Q., et al. Evidence-based toxicity evaluation and scheduling of Chinese herbal medicines. Journal of Ethnopharmacology . 2013;146(1):40–61. - PubMed
-
- Cosyns J. P. Aristolochic acid and ‘Chinese herbs nephropathy’: a review of the evidence to date. Drug Safety . 2003;26(1):33–48. - PubMed
-
- Zhang Z. B., Jiang W. Y., Cai S. Q. Clinical and pharmaceutical development and its thought of Chinese herbal nephropathy induced by aristolochic acid. Chinese Traditional and Herbal Drugs . 2003;34(2):93–96.
LinkOut - more resources
Full Text Sources
Research Materials