Effect of 0.01% atropine eyedrops on intraocular pressure in schoolchildren: a randomized clinical trial
- PMID: 36124178
- PMCID: PMC9453406
- DOI: 10.18240/ijo.2022.09.04
Effect of 0.01% atropine eyedrops on intraocular pressure in schoolchildren: a randomized clinical trial
Abstract
Aim: To assess the effect of 0.01% atropine eye drops on intraocular pressure (IOP) in myopic children.
Methods: A placebo-controlled, double-masked, randomized study. Totally 220 children aged 6 to 12y with myopia ranging from -1.00 to -6.00 D in both eyes were enrolled. Children were randomized in a 1:1 ratio to either 0.01% atropine eye drops or a placebo group using generated random numbers. All participants underwent the examination of IOP and cycloplegic refraction at baseline, 6 and 12mo. The change of IOP and the proportion of subjects with increased IOP in atropine and placebo groups were compared.
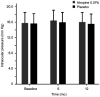
Results: Of 220 children, 117 were boys (53.2%). A total of 159 (72.3%) participants completed the follow-up at the 1-year study. At baseline, the mean IOP was 15.74 mm Hg (95%CI, 15.13 to 16.34 mm Hg) for the 0.01% atropine group and 15.59 mm Hg (95%CI, 15.00 to 16.19 mm Hg) for placebo group (mean difference, 0.14 mm Hg; P=0.743) after adjusting for central corneal thickness at baseline. At one year follow-up, the mean change of IOP was 0.16 mm Hg (95%CI, -0.43 to 0.76 mm Hg) for the 0.01% atropine group and -0.11 mm Hg (95%CI, -0.71 to 0.50 mm Hg) for placebo group (mean difference, 0.27 mm Hg; P=0.525) after adjusting for central corneal thickness. The 51.4% of children have increased IOP in the 0.01% atropine group, compared with 45.9% in the placebo group (P=0.511).
Conclusion: The 0.01% atropine eye drops do not significantly affect the risk of elevated IOP. It is relatively safer to use in the studies that try to minimize myopia progression. However, a further long-duration study is required to be validated.
Keywords: 0.01% atropine eye drops; intraocular pressure; myopic children.
International Journal of Ophthalmology Press.
Figures
References
-
- Baird PN, Saw SM, Lanca C, et al. Myopia. Nat Rev Dis Primers. 2020;6(1):99. - PubMed
-
- Weiss RS, Park S. Recent updates on myopia control: preventing progression 1 diopter at a time. Curr Opin Ophthalmol. 2019;30(4):215–219. - PubMed
-
- Morgan IG, French AN, Ashby RS, Guo XX, Ding XH, He MG, Rose KA. The epidemics of myopia: aetiology and prevention. Prog Retin Eye Res. 2018;62:134–149. - PubMed
-
- Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P, Wong TY, Naduvilath TJ, Resnikoff S. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123(5):1036–1042. - PubMed
-
- Li SM, Liu LR, Li SY, et al. Design, methodology and baseline data of a school-based cohort study in central China: the Anyang childhood eye study. Ophthalmic Epidemiol. 2013;20(6):348–359. - PubMed
LinkOut - more resources
Full Text Sources