Comparison of intravitreal aflibercept and dexamethasone implant in the treatment of macular edema associated with diabetic retinopathy or retinal vein occlusion: a Meta-analysis and systematic review
- PMID: 36124196
- PMCID: PMC9453398
- DOI: 10.18240/ijo.2022.09.15
Comparison of intravitreal aflibercept and dexamethasone implant in the treatment of macular edema associated with diabetic retinopathy or retinal vein occlusion: a Meta-analysis and systematic review
Abstract
Aim: To compare the efficacy and safety of intravitreal aflibercept with dexamethasone implant in the treatment of macular edema (ME) associated with diabetic retinopathy (DR) or retinal vein occlusion (RVO).
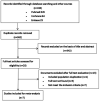
Methods: A comprehensive search of studies comparing dexamethasone and aflibercept in patients with ME was conducted at PubMed, Embase, and Cochrane Central Register of Controlled Trials from the beginning of library to April 16, 2021. Extracting the data including best-corrected visual acuity (BCVA), central retinal thickness (CRT), number of injections and serious adverse events (SAEs) from the final qualified articles. RevMan 5.3 software was used for Meta-analysis of the included studies.
Results: Totally 7 studies with 369 eyes were included. The causes of ME in the final screening study included RVO and DR. Compared with the aflibercept treatment group, the BCVA of the dexamethasone implant treatment group showed no significant difference in the follow-up for 3mo [mean difference (MD): -0.05, 95% confidence interval (CI): -0.11, 0.02; P=0.17] and 12mo (MD: -0.01, 95%CI: -0.38, 0.37; P=0.98), but it was slightly worse than the aflibercept group at 6mo (MD: 0.12, 95%CI: 0.03, 0.21; P=0.008). In terms of CRT reduction, there was no significant difference between the two groups at 3mo (MD: -28.14, 95%CI: -79.95, 23.67; P=0.29), 6mo (MD: 27.67, 95%CI: -84.89, 140.24; P=0.63), and 12mo (MD: -59.00, 95%CI: -127.37, 9.37; P=0.09). However, dexamethasone implant had fewer injections, but more adverse events such as elevated intraocular pressure (IOP) and cataract.
Conclusion: Intravitreal injection of aflibercept and dexamethasone implant can both effectively increase BCVA and reduce CRT. Compared with aflibercept, dexamethasone implant is not inferior in improving vision and reducing CRT in the initial treatment period (3mo) and long-term treatment period (12mo). Besides, it has fewer injections and more likely to cause elevated IOP and cataract.
Keywords: Meta-analysis; aflibercept; best-corrected visual acuity; central retinal thickness; dexamethasone; macular edema.
International Journal of Ophthalmology Press.
Figures



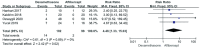


Similar articles
-
Efficacy and safety of dexamethasone versus intravitreal aflibercept implants for macular edema: a systematic review and meta-analysis.Eur J Med Res. 2025 Apr 15;30(1):273. doi: 10.1186/s40001-025-02404-x. Eur J Med Res. 2025. PMID: 40229845 Free PMC article.
-
Comparing the efficacy of dexamethasone implant and anti-VEGF for the treatment of macular edema: A systematic review and meta-analysis.PLoS One. 2024 Jul 10;19(7):e0305573. doi: 10.1371/journal.pone.0305573. eCollection 2024. PLoS One. 2024. PMID: 38985778 Free PMC article.
-
Anti-vascular endothelial growth factor for macular oedema secondary to branch retinal vein occlusion.Cochrane Database Syst Rev. 2020 Jul 7;7(7):CD009510. doi: 10.1002/14651858.CD009510.pub3. Cochrane Database Syst Rev. 2020. PMID: 32633861 Free PMC article.
-
Dexamethasone implant for refractory macular edema secondary to diabetic retinopathy and retinal vein occlusion.Int J Ophthalmol. 2024 Oct 18;17(10):1837-1842. doi: 10.18240/ijo.2024.10.09. eCollection 2024. Int J Ophthalmol. 2024. PMID: 39430031 Free PMC article.
-
Simultaneous intravitreal dexamethasone and aflibercept for refractory macular edema secondary to retinal vein occlusion.Graefes Arch Clin Exp Ophthalmol. 2020 Apr;258(4):787-793. doi: 10.1007/s00417-019-04577-8. Epub 2020 Jan 2. Graefes Arch Clin Exp Ophthalmol. 2020. PMID: 31897703
Cited by
-
Efficacy and safety of dexamethasone versus intravitreal aflibercept implants for macular edema: a systematic review and meta-analysis.Eur J Med Res. 2025 Apr 15;30(1):273. doi: 10.1186/s40001-025-02404-x. Eur J Med Res. 2025. PMID: 40229845 Free PMC article.
-
Pars plana suturing of intravitreal fluocinolone acetonide in noninfectious uveitis.Graefes Arch Clin Exp Ophthalmol. 2025 Jul 10. doi: 10.1007/s00417-025-06901-x. Online ahead of print. Graefes Arch Clin Exp Ophthalmol. 2025. PMID: 40637804
-
Comparing the efficacy of dexamethasone implant and anti-VEGF for the treatment of macular edema: A systematic review and meta-analysis.PLoS One. 2024 Jul 10;19(7):e0305573. doi: 10.1371/journal.pone.0305573. eCollection 2024. PLoS One. 2024. PMID: 38985778 Free PMC article.
-
Optical coherence tomography angiography for macular microvessels in ischemic branch retinal vein occlusion treated with conbercept: predictive factors for the prognosis.Int J Ophthalmol. 2023 Dec 18;16(12):2049-2055. doi: 10.18240/ijo.2023.12.18. eCollection 2023. Int J Ophthalmol. 2023. PMID: 38111937 Free PMC article.
-
Dexamethasone intravitreal implant monotherapy in naive patients with macular edema secondary to retinal vein occlusion: long term follow-up retrospective cohort study.Int J Ophthalmol. 2025 May 18;18(5):876-882. doi: 10.18240/ijo.2025.05.13. eCollection 2025. Int J Ophthalmol. 2025. PMID: 40385126 Free PMC article.
References
-
- Ferris FL, 3rd, Patz A. Macular edema. A complication of diabetic retinopathy. Surv Ophthalmol. 1984;28 Suppl:452–461. - PubMed
-
- Patz A, Schatz H, Berkow JW, Gittelsohn AM, Ticho U. Macular edema—an overlooked complication of diabetic retinopathy. Trans Am Acad Ophthalmol Otolaryngol. 1973;77(1):OP34–OP42. - PubMed
-
- Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin epidemiologic study of diabetic retinopathy. XV. The long-term incidence of macular edema. Ophthalmology. 1995;102(1):7–16. - PubMed
-
- Ting DSW, Cheung GCM, Wong TY. Diabetic retinopathy: global prevalence, major risk factors, screening practices and public health challenges: a review. Clin Exp Ophthalmol. 2016;44(4):260–277. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
