P2X7R-NEK7-NLRP3 Inflammasome Activation: A Novel Therapeutic Pathway of Qishen Granule in the Treatment of Acute Myocardial Ischemia
- PMID: 36124207
- PMCID: PMC9482414
- DOI: 10.2147/JIR.S373962
P2X7R-NEK7-NLRP3 Inflammasome Activation: A Novel Therapeutic Pathway of Qishen Granule in the Treatment of Acute Myocardial Ischemia
Abstract
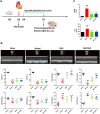
Background: Acute myocardial ischemia (AMI) is a common heart disease with increasing morbidity and mortality year by year. Persistent and sterile inflammatory infiltration of myocardial tissue is an important factor triggering of acute myocardial ischemia secondary to acute myocardial infarction, and NLRP3 inflammasome activation is an important part of sterile inflammatory response after acute myocardial ischemia. Previous studies have shown that Qishen granule (QSG) can significantly inhibit the inflammatory injury of myocardial tissue caused by ischemia, but its effect and specific mechanism of inhibiting the activation of NLRP3 inflammasome have not been reported. This study was to investigate the specific mechanism of QSG inhibiting inflammation after AMI, and to validate the possible targets.
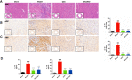
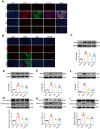
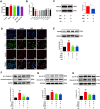
Methods: The myocardial ischemia model in mice was established by ligation of the left anterior descending coronary artery. Echocardiography was used to evaluate the cardiac function of the mice. Plasma CK-MB and cTnl were detected by ELISA to evaluate the degree of myocardial injury. The extent of myocardial tissue inflammation in mice was assessed by HE staining and immunohistochemistry of IL-18, IL-1β. The expressions of NLRP3, ASC, Caspase-1, and CD86 were detected by immunofluorescence; detection of key pathway proteins P2X7R, NEK7, NLRP3, ASC, Caspase-1, and effector proteins IL-18, IL-1β by Western blot. In vitro experiments, ATP+LPS was used to construct a RAW264.7 macrophage NLRP3 inflammasome activation model. Immunofluorescence and Western blot analysis were performed to detect the expression of NLRP3 pathway activator and effector proteins. Plasmid-transfected P2X7R overexpression and immunoprecipitation assays were used to evaluate the QSG-regulated NLRP3 inflammasome activation pathway.
Results: QSG rescued cardiac function and further reduced inflammatory effects in mice by inhibiting NLRP3 inflammasome activation. In vitro, QSG inhibited LPS combined with ATP-induced NLRP3 inflammasome activation in RAW264.7 macrophages by downregulating the expression of NLRP3 inflammasome key pathway proteins. In addition, inhibition or overexpression of P2X7R in RAW264.7 macrophages and immunoprecipitated protein interactions further confirmed that QSG reduces macrophages inflammasome activation via the P2X7R-NEK7-NLRP3 pathway.
Conclusion: P2X7R-NEK7-NLRP3 inflammasome activation is a novel therapeutic mechanism of QSG in the treatment of acute myocardial ischemia.
Keywords: NLRP3 inflammasome; P2X7R-NEK7; Qishen granule; acute myocardial ischemia; inflammation; macrophages.
© 2022 Li et al.
Conflict of interest statement
The authors report no conflicts of interest.
Figures


Similar articles
-
Qishen granule (QSG) exerts cardioprotective effects by inhibiting NLRP3 inflammasome and pyroptosis in myocardial infarction rats.J Ethnopharmacol. 2022 Mar 1;285:114841. doi: 10.1016/j.jep.2021.114841. Epub 2021 Nov 16. J Ethnopharmacol. 2022. PMID: 34793884
-
Dan-Lou tablets reduce inflammatory response by inhibiting the activation of NLRP3 inflammasome for coronary heart disease.Phytomedicine. 2024 Aug;131:155773. doi: 10.1016/j.phymed.2024.155773. Epub 2024 May 27. Phytomedicine. 2024. PMID: 38833946
-
Electroacupuncture preconditioning attenuates acute myocardial ischemia injury through inhibiting NLRP3 inflammasome activation in mice.Life Sci. 2020 May 1;248:117451. doi: 10.1016/j.lfs.2020.117451. Epub 2020 Feb 20. Life Sci. 2020. PMID: 32088213
-
Estrogen plays an important role by influencing the NLRP3 inflammasome.Biomed Pharmacother. 2023 Nov;167:115554. doi: 10.1016/j.biopha.2023.115554. Epub 2023 Sep 20. Biomed Pharmacother. 2023. PMID: 37738797 Review.
-
Research progress on the improvement of cardiovascular diseases through the autonomic nervous system regulation of the NLRP3 inflammasome pathway.Front Cardiovasc Med. 2024 Apr 8;11:1369343. doi: 10.3389/fcvm.2024.1369343. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 38650918 Free PMC article. Review.
Cited by
-
Run-Mu-Ling Granules Mitigate Ocular Surface Inflammatory Injury Associated with Dry Eye by Suppressing the NLRP3/GSDMD-Mediated Pyroptosis Pathway.J Inflamm Res. 2024 Dec 10;17:10769-10784. doi: 10.2147/JIR.S496231. eCollection 2024. J Inflamm Res. 2024. PMID: 39677285 Free PMC article.
-
High-Density Lipoproteins at the Interface between the NLRP3 Inflammasome and Myocardial Infarction.Int J Mol Sci. 2024 Jan 20;25(2):1290. doi: 10.3390/ijms25021290. Int J Mol Sci. 2024. PMID: 38279290 Free PMC article. Review.
-
Qishen Granules Modulate Metabolism Flexibility Against Myocardial Infarction via HIF-1 α-Dependent Mechanisms in Rats.Chin J Integr Med. 2025 Mar;31(3):215-227. doi: 10.1007/s11655-024-3667-y. Epub 2024 Sep 21. Chin J Integr Med. 2025. PMID: 39305458
-
Role of pyroptosis in diabetic cardiomyopathy: an updated review.Front Endocrinol (Lausanne). 2024 Jan 5;14:1322907. doi: 10.3389/fendo.2023.1322907. eCollection 2023. Front Endocrinol (Lausanne). 2024. PMID: 38250736 Free PMC article. Review.
-
P2X7R Modulates NEK7-NLRP3 Interaction to Exacerbate Experimental Autoimmune Prostatitis via GSDMD-mediated Prostate Epithelial Cell Pyroptosis.Int J Biol Sci. 2024 Jun 11;20(9):3393-3411. doi: 10.7150/ijbs.94704. eCollection 2024. Int J Biol Sci. 2024. PMID: 38993566 Free PMC article.
References
-
- Barstow C. Acute coronary syndrome: presentation and diagnostic evaluation. FP Essent. 2020;490:11–19. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

