Light quality as a driver of photosynthetic apparatus development
- PMID: 36124269
- PMCID: PMC9481803
- DOI: 10.1007/s12551-022-00985-z
Light quality as a driver of photosynthetic apparatus development
Abstract
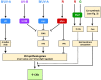
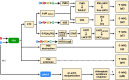
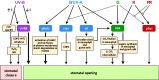
Light provides energy for photosynthesis and also acts as an important environmental signal. During their evolution, plants acquired sophisticated sensory systems for light perception and light-dependent regulation of their growth and development in accordance with the local light environment. Under natural conditions, plants adapted by using their light sensors to finely distinguish direct sunlight and dark in the soil, deep grey shade under the upper soil layer or litter, green shade under the canopy and even lateral green reflectance from neighbours. Light perception also allows plants to evaluate in detail the weather, time of day, day length and thus the season. However, in artificial lighting conditions, plants are confronted with fundamentally different lighting conditions. The advent of new light sources - light-emitting diodes (LEDs), which emit narrow-band light - allows growing plants with light of different spectral bands or their combinations. This sets the task of finding out how light of different quality affects the development and functioning of plants, and in particular, their photosynthetic apparatus (PSA), which is one of the basic processes determining plant yield. In this review, we briefly describe how plants perceive environment light signals by their five families of photoreceptors and by the PSA as a particular light sensor, and how they use this information to form their PSA under artificial narrow-band LED-based lighting of different spectral composition. We consider light regulation of the biosynthesis of photosynthetic pigments, photosynthetic complexes and chloroplast ATP synthase function, PSA photoprotection mechanisms, carbon assimilation reactions and stomatal development and function.
Keywords: LED lighting; Light quality; Photosynthetic apparatus regulation; Photosynthetic carbon assimilation; Photosynthetic pigment synthesis; Stomata.
© International Union for Pure and Applied Biophysics (IUPAB) and Springer-Verlag GmbH Germany, part of Springer Nature 2022.
Conflict of interest statement
Conflict of interestThe authors declare no competing interests.
Figures



Similar articles
-
Leaf gas exchange, chlorophyll fluorescence and pigment indexes of Eugenia uniflora L. in response to changes in light intensity and soil flooding.Tree Physiol. 2010 Jan;30(1):45-55. doi: 10.1093/treephys/tpp095. Epub 2009 Nov 18. Tree Physiol. 2010. PMID: 19923194
-
Effects of Light Spectral Quality on the Micropropagated Raspberry Plants during Ex Vitro Adaptation.Plants (Basel). 2021 Sep 30;10(10):2071. doi: 10.3390/plants10102071. Plants (Basel). 2021. PMID: 34685878 Free PMC article.
-
Selection of LED lighting systems for the reduction of the biodeterioration of speleothems induced by photosynthetic biofilms in the Nerja Cave (Malaga, Spain).J Photochem Photobiol B. 2021 Apr;217:112155. doi: 10.1016/j.jphotobiol.2021.112155. Epub 2021 Feb 17. J Photochem Photobiol B. 2021. PMID: 33640830
-
Spectral signatures of photosynthesis. I. Review of Earth organisms.Astrobiology. 2007 Feb;7(1):222-51. doi: 10.1089/ast.2006.0105. Astrobiology. 2007. PMID: 17407409 Review.
-
[Regulation of photosynthesis by light quality and its mechanism in plants].Ying Yong Sheng Tai Xue Bao. 2008 Jul;19(7):1619-24. Ying Yong Sheng Tai Xue Bao. 2008. PMID: 18839928 Review. Chinese.
Cited by
-
Effect of Interactions between Phosphorus and Light Intensity on Metabolite Compositions in Tea Cultivar Longjing43.Int J Mol Sci. 2022 Dec 2;23(23):15194. doi: 10.3390/ijms232315194. Int J Mol Sci. 2022. PMID: 36499516 Free PMC article.
-
Biophysical Reviews: Turning the page from 2022 to 2023.Biophys Rev. 2023 Feb 23;15(1):1-11. doi: 10.1007/s12551-023-01049-6. eCollection 2023 Feb. Biophys Rev. 2023. PMID: 36909962 Free PMC article.
-
The effect of LED light quality on the carotenoid metabolism and related gene expression in the genus Brassica.BMC Plant Biol. 2023 Jun 21;23(1):328. doi: 10.1186/s12870-023-04326-4. BMC Plant Biol. 2023. PMID: 37340342 Free PMC article.
-
Light Quality Plays a Crucial Role in Regulating Germination, Photosynthetic Efficiency, Plant Development, Reactive Oxygen Species Production, Antioxidant Enzyme Activity, and Nutrient Acquisition in Alfalfa.Int J Mol Sci. 2025 Jan 3;26(1):360. doi: 10.3390/ijms26010360. Int J Mol Sci. 2025. PMID: 39796215 Free PMC article.
-
Performance of the Photosynthetic Apparatus under Glass with a Luminophore Modifying Red-To-Far-Red-Light Ratio-A Case Study.Cells. 2023 Jun 5;12(11):1552. doi: 10.3390/cells12111552. Cells. 2023. PMID: 37296672 Free PMC article.
References
-
- Akhtar P, Görföl F, Garab G, Lambrev PH. Dependence of chlorophyll fluorescence quenching on the lipid-to-protein ratio in reconstituted light-harvesting complex II membranes containing lipid labels. Chem Phys. 2019;522:242–248. doi: 10.1016/j.chemphys.2019.03.012. - DOI
-
- Amoozgar A, Mohammadi A, Sabzalian MR. Impact of light-emitting diode irradiation on photosynthesis, phytochemical composition and mineral element content of lettuce cv. Grizzly. Photosynthetica. 2017;55(1):85–95. doi: 10.1007/s11099-016-0216-8. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

