In vitro skeletal muscle models for type 2 diabetes
- PMID: 36124295
- PMCID: PMC9478902
- DOI: 10.1063/5.0096420
In vitro skeletal muscle models for type 2 diabetes
Abstract
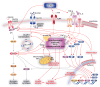
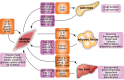
Type 2 diabetes mellitus, a metabolic disorder characterized by abnormally elevated blood sugar, poses a growing social, economic, and medical burden worldwide. The skeletal muscle is the largest metabolic organ responsible for glucose homeostasis in the body, and its inability to properly uptake sugar often precedes type 2 diabetes. Although exercise is known to have preventative and therapeutic effects on type 2 diabetes, the underlying mechanism of these beneficial effects is largely unknown. Animal studies have been conducted to better understand the pathophysiology of type 2 diabetes and the positive effects of exercise on type 2 diabetes. However, the complexity of in vivo systems and the inability of animal models to fully capture human type 2 diabetes genetics and pathophysiology are two major limitations in these animal studies. Fortunately, in vitro models capable of recapitulating human genetics and physiology provide promising avenues to overcome these obstacles. This review summarizes current in vitro type 2 diabetes models with focuses on the skeletal muscle, interorgan crosstalk, and exercise. We discuss diabetes, its pathophysiology, common in vitro type 2 diabetes skeletal muscle models, interorgan crosstalk type 2 diabetes models, exercise benefits on type 2 diabetes, and in vitro type 2 diabetes models with exercise.
© 2022 Author(s).
Conflict of interest statement
The authors have no conflicts to disclose.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
