Antibody profile in post-vaccinated & SARS-CoV-2 infected individuals
- PMID: 36124500
- PMCID: PMC9807196
- DOI: 10.4103/ijmr.ijmr_3330_21
Antibody profile in post-vaccinated & SARS-CoV-2 infected individuals
Abstract
Background & objectives: During the COVID-19 pandemic it was important to assess the antibody profile in individuals vaccinated with Covaxin (BBV152) and Covishield (ChAdOx1 nCoV-19) with both 28 and 84 days gaps between two doses, those infected with SARS-CoV-2 and post-COVID-19-infected individuals vaccinated with only one dose of either of the vaccines. The present study was aimed to assess these objectives.
Methods: Fifty real time reverse transcription-polymerase chain reaction (qRT-PCR)-confirmed COVID-19-infected individuals, along with 90 COVID-19-naïve (BBV152 and ChAdOx1 nCov-19)-vaccinated individuals, were included in the study. Individuals who received a single dose of either vaccine with a confirmed past diagnosis of SARS-CoV-2 infection (n=15) were also included. Blood samples were collected strictly between the 4th and 5th wk after development of symptoms for SARS-CoV-2 infected individuals and after the first/second vaccination dose. Antibody profile assessment was done using whole-virus, spike-receptor binding domain (RBD) and nucleocapsid-specific ELISA kits along with neutralizing antibody kit.
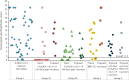
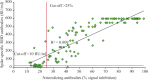
Results: There was an overall 97.7 per cent seropositivity rate in vaccinated individuals, and a strong correlation (R2=0.8, P<0.001) between neutralizing and spike-RBD antibodies. Among individuals who received two standard doses of ChAdOx1 nCoV-19 vaccine, the spike antibody levels developed were of higher titre with a longer prime boost interval than in those with shorter intervals (P<0.01). Individuals vaccinated with two doses as well as only one dose post-SARS-CoV-2 infection had high neutralizing and spike-specific antibodies.
Interpretation & conclusions: High neutralizing and spike-specific antibodies were developed in individuals vaccinated only with one dose of either vaccine post-SARS-CoV-2 infection. With the main priority being vaccinating majority of the population in our country, single-dose administration to such individuals would be a sensible way to make the most of the limited supplies. Furthermore, neutralizing antibody levels observed in COVID-19-naïve vaccinees imply the need for booster vaccination.
Keywords: BBV152; ChAdOx1 nCov-19; SARS-CoV-2 infection; neutralizing antibody; spike-specific antibody; vaccination.
Conflict of interest statement
Figures


References
-
- Voysey M, Costa Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine:A pooled analysis of four randomised trials. Lancet. 2021;397:881–91. - PMC - PubMed
-
- Bharat Biotech announces phase 3 results of COVAXIN®:India's first COVID-19 vaccine demonstrates interim clinical efficacy of 81%. [accessed on May 31, 2021]. Available from: https://www.bharatbiotech.com/images/press/covaxin-phase3-efficacy-resul... .
-
- Ella R, Reddy S, Jogdand H, Sarangi V, Ganneru B, Prasad S, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152:Interim results from a double-blind, randomised, multicentre, phase 2 trial, and 3-month follow-up of a double-blind, randomised phase 1 trial. Lancet Infect Dis. 2021;21:950–61. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
