Pathogen inactivation treatment of triple-dose apheresis platelets with amotosalen and ultraviolet a light
- PMID: 36124649
- PMCID: PMC10087429
- DOI: 10.1111/tme.12913
Pathogen inactivation treatment of triple-dose apheresis platelets with amotosalen and ultraviolet a light
Abstract
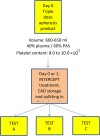
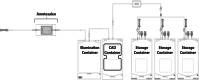
Background: A triple storage (TS) set allows for pathogen inactivation (PI) treatment of triple-dose apheresis platelet products with amotosalen + UVA. We evaluated the quality and metabolic parameters of platelet concentrates (PCs) pathogen inactivated and stored for 7 days.
Materials and methods: Twelve triple-dose products collected with two different apheresis platforms were treated with amotosalen+UVA. Products were split into three single-dose units. Testing was made pretreatment, after splitting, at days 5 and 7 of storage.
Results: Single-dose PI PCs had a mean platelet content of 2.89 ± 0.35 x 1011 . From baseline to day 7, pH remained stable (7.1 ± 0.1 vs. 7.0 ± 0.1), pO2 increased (11.3 ± 2.4 vs. 18.3 ± 3.5 kPa) as did LDH (201 ± 119 vs. 324 ± 203 U/L) and lactate (3.6 ± 1.7 vs. 12.1 ± 1.5 mmol/L) (all p < 0.01); pCO2 decreased (4.1 ± 0.8 vs. 1.5 ± 0.7 mmHg; p < 0.01) and so did bicarbonate (6.6 ± 1.1 vs. 2.5 ± 1.4 mmol/L), glucose (5.6 ± 1.2 vs. 0.4 ± 0.4 mmol/L) and ATP (3.4 ± 0.9 vs. 2.5 ± 1.4 nmol/108 platelets) (all p < 0.05).
Conclusion: Triple-dose PCs processed with the TS sets fulfilled the quality requirements and displayed metabolic changes of expected extent during 7-day storage.
Keywords: pathogen inactivation; triple-dose apheresis platelet components.
© 2022 The Authors. Transfusion Medicine published by John Wiley & Sons Ltd on behalf of British Blood Transfusion Society.
Conflict of interest statement
Silke Andresen, Jean‐Marc Payrat, and Jin‐Sying Lin are employees of Cerus Corporation.
Figures
References
-
- Sandgren P, Diedrich B. Pathogen inactivation of double‐dose buffy‐coat platelet concentrates photochemically treated with amotosalen and UVA light: preservation of in vitro function. Vox Sang. 2015;108:340‐349. - PubMed
-
- Ohlsson S, Diedrich B, Uhlin M, Sandgren P. Optimized processing for pathogen inactivation of double‐dose buffy‐coat platelet concentrates: maintained in vitro quality over 7‐day storage. Vox Sang. 2018;113:611‐621. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

