Spatial organization of neuron-astrocyte interactions in the somatosensory cortex
- PMID: 36124663
- PMCID: PMC10110431
- DOI: 10.1093/cercor/bhac357
Spatial organization of neuron-astrocyte interactions in the somatosensory cortex
Abstract
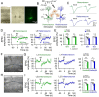
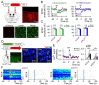
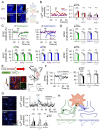
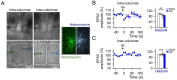
Microcircuits in the neocortex are functionally organized along layers and columns, which are the fundamental modules of cortical information processing. While the function of cortical microcircuits has focused on neuronal elements, much less is known about the functional organization of astrocytes and their bidirectional interaction with neurons. Here, we show that Cannabinoid type 1 receptor (CB1R)-mediated astrocyte activation by neuron-released endocannabinoids elevate astrocyte Ca2+ levels, stimulate ATP/adenosine release as gliotransmitters, and transiently depress synaptic transmission in layer 5 pyramidal neurons at relatively distant synapses (˃20 μm) from the stimulated neuron. This astrocyte-mediated heteroneuronal synaptic depression occurred between pyramidal neurons within a cortical column and was absent in neurons belonging to adjacent cortical columns. Moreover, this form of heteroneuronal synaptic depression occurs between neurons located in particular layers, following a specific connectivity pattern that depends on a layer-specific neuron-to-astrocyte signaling. These results unravel the existence of astrocyte-mediated nonsynaptic communication between cortical neurons and that this communication is column- and layer-specific, which adds further complexity to the intercellular signaling processes in the neocortex.
Keywords: astrocyte; calcium imaging; cortex; neuron-astrocyte communication; synaptic transmission.
© The Author(s) 2022. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


References
-
- Alger BE. Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. Prog Neurobiol. 2002:68(4):247–286. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

