Reporting and management of incidental lung findings on computed tomography: beyond lung nodules
- PMID: 36124681
- PMCID: PMC9975526
- DOI: 10.1259/bjr.20220207
Reporting and management of incidental lung findings on computed tomography: beyond lung nodules
Abstract
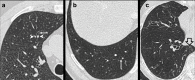
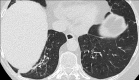
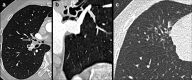
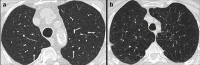
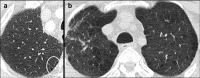
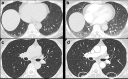
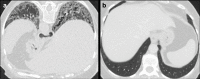
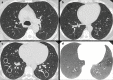
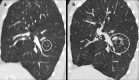
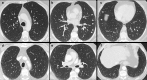
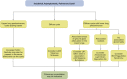
Non-nodular incidental lung findings can broadly be categorised as airway- or airspace-related abnormalities and diffuse parenchymal abnormalities. Airway-related abnormalities include bronchial dilatation and thickening, foci of low attenuation, emphysema, and congenital variants. Diffuse parenchymal abnormalities relate to the spectrum of diffuse parenchymal lung diseases cover a spectrum from interstitial lung abnormalities (ILAs) and pulmonary cysts to established diffuse parenchymal lung abnormalities such as the idiopathic interstitial pneumonias and cystic lung diseases. In this review, we discuss the main manifestations of these incidental findings, paying attention to their prevalence and importance, descriptors to use when reporting, the limits of what can be considered "normal", and conclude each section with some pragmatic reporting recommendations. We also highlight technical and patient factors which can lead to spurious abnormalities.
Figures


References
-
- Hatabu H, Hunninghake GM, Richeldi L, Brown KK, Wells AU, Remy-Jardin M, et al. . Interstitial lung abnormalities detected incidentally on CT: a position paper from the fleischner society. Lancet Respir Med 2020; 8: 726–37: S2213-2600(20)30168-5. doi: 10.1016/S2213-2600(20)30168-5 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

