Comparative analysis of tangential flow filtration and ultracentrifugation, both combined with subsequent size exclusion chromatography, for the isolation of small extracellular vesicles
- PMID: 36124834
- PMCID: PMC9486818
- DOI: 10.1002/jev2.12266
Comparative analysis of tangential flow filtration and ultracentrifugation, both combined with subsequent size exclusion chromatography, for the isolation of small extracellular vesicles
Abstract
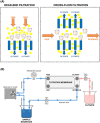
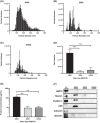
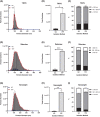
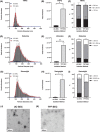
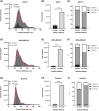
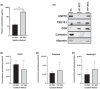
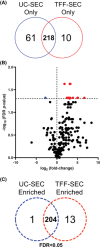
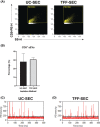
Small extracellular vesicles (sEVs) provide major promise for advances in cancer diagnostics, prognostics, and therapeutics, ascribed to their distinctive cargo reflective of pathophysiological status, active involvement in intercellular communication, as well as their ubiquity and stability in bodily fluids. As a result, the field of sEV research has expanded exponentially. Nevertheless, there is a lack of standardisation in methods for sEV isolation from cells grown in serum-containing media. The majority of researchers use serum-containing media for sEV harvest and employ ultracentrifugation as the primary isolation method. Ultracentrifugation is inefficient as it is devoid of the capacity to isolate high sEV yields without contamination of non-sEV materials or disruption of sEV integrity. We comprehensively evaluated a protocol using tangential flow filtration and size exclusion chromatography to isolate sEVs from a variety of human and murine cancer cell lines, including HeLa, MDA-MB-231, EO771 and B16F10. We directly compared the performance of traditional ultracentrifugation and tangential flow filtration methods, that had undergone further purification by size exclusion chromatography, in their capacity to separate sEVs, and rigorously characterised sEV properties using multiple quantification devices, protein analyses and both image and nano-flow cytometry. Ultracentrifugation and tangential flow filtration both enrich consistent sEV populations, with similar size distributions of particles ranging up to 200 nm. However, tangential flow filtration exceeds ultracentrifugation in isolating significantly higher yields of sEVs, making it more suitable for large-scale research applications. Our results demonstrate that tangential flow filtration is a reliable and robust sEV isolation approach that surpasses ultracentrifugation in yield, reproducibility, time, costs and scalability. These advantages allow for implementation in comprehensive research applications and downstream investigations.
Keywords: cell culture; extracellular vesicles; isolation; tangential flow filtration.
© 2022 The Authors. Journal of Extracellular Vesicles published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles.
Conflict of interest statement
The authors report no conflict of interest. This work was funded by grants from the National Health and Medical Research Council Australia (APP1185907) and National Breast Cancer Foundation Australia (IIRS‐18‐159) to A.M. C.S. is supported by The National Health and Medical Research Council (NHMRC, 1114013).
Figures

References
-
- Alameldin, S. , Costina, V. , Abdel‐Baset, H. A. , Nitschke, K. , Nuhn, P. , Neumaier, M. , & Hedtke, M. (2021). Coupling size exclusion chromatography to ultracentrifugation improves detection of exosomal proteins from human plasma by LC‐MS. Practical Laboratory Medicine, 26, e00241. - PMC - PubMed
-
- Anderson, W. , Kozak, D. , Coleman, V. A. , Jämting, Å. K. , & Trau, M. (2013). A comparative study of submicron particle sizing platforms: Accuracy, precision and resolution analysis of polydisperse particle size distributions. Journal of Colloid and Interface Science, 405, 322–330. - PubMed
-
- Andrade, A. C. , Wolf, M. , Binder, H.‐M. , Gomes, F. G. , Manstein, F. , Ebner‐Peking, P. , Poupardin, R. , Zweigerdt, R. , Schallmoser, K. , & Strunk, D. (2021). Hypoxic conditions promote the angiogenic potential of human induced pluripotent stem cell‐derived extracellular vesicles. International Journal of Molecular Sciences, 22(8), 3890. - PMC - PubMed
-
- Arab, T. , Mallick, E. R. , Huang, Y. , Dong, L. , Liao, Z. , Zhao, Z. , Gololobova, O. , Smith, B. , Haughey, N. J. , Pienta, K. J. , Slusher, B. S. , Tarwater, P. M. , Tosar, J. P. , Zivkovic, A. M. , Vreeland, W. N. , Paulaitis, M. E. , & Witwer, K. W. (2021). Characterization of extracellular vesicles and synthetic nanoparticles with four orthogonal single‐particle analysis platforms. Journal of Extracellular Vesicles, 10(6), e12079. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

