Second-Generation Human Immunodeficiency Virus Integrase Inhibitors Induce Differentiation Dysregulation and Exert Toxic Effects in Human Embryonic Stem Cell and Mouse Models
- PMID: 36124861
- PMCID: PMC10205620
- DOI: 10.1093/infdis/jiac386
Second-Generation Human Immunodeficiency Virus Integrase Inhibitors Induce Differentiation Dysregulation and Exert Toxic Effects in Human Embryonic Stem Cell and Mouse Models
Abstract
Background: Each year, approximately 1.1 million children are exposed in utero to human immunodeficiency virus antiretrovirals, yet their safety is often not well characterized during pregnancy. The Tsepamo study reported a neural tube defect signal in infants exposed to the integrase strand transfer inhibitor (InSTI) dolutegravir from conception, suggesting that exposure during early fetal development may be detrimental.
Methods: The effects of InSTIs on 2 human embryonic stem cell (hESC) lines were characterized with respect to markers of pluripotency, early differentiation, and cellular health. In addition, fetal resorptions after exposure to InSTIs from conception were analyzed in pregnant mice.
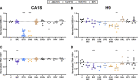
Results: At subtherapeutic concentrations, second-generation InSTIs bictegravir, cabotegravir, and dolutegravir decreased hESC counts and pluripotency and induced dysregulation of genes involved in early differentiation. At therapeutic concentrations, bictegravir induced substantial hESC death and fetal resorptions. It is notable that first-generation InSTI raltegravir did not induce any hESC toxicity or differentiation, at any concentration tested.
Conclusions: Exposure to some InSTIs, even at subtherapeutic concentrations, can induce adverse effects in hESCs and pregnant mice. Given the increasingly prevalent use of second-generation InSTIs, including in women of reproductive age, it is imperative to further elucidate the effect of InSTIs on embryonic development, as well as their long-term safety after in utero exposure.
Keywords: HIV; antiretroviral; hESC; integrase inhibitor; pregnancy.
© The Author(s) 2022. Published by Oxford University Press on behalf of Infectious Diseases Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures




References
-
- UNAIDS . AIDSInfo 2020. 2020. https://aidsinfo.unaids.org. Accessed 18 October 2021.
-
- Zash RM. What will it take to refute the possible safety signal for dolutegravir and neural tube defects? BJOG-Int J Obstet Gy 2019; 126:1346. - PubMed
-
- World Health Organization . Update of recommendations on first- and second-line antiretroviral regimens. 2019. https://www.who.int/publications/i/item/WHO-CDS-HIV-19.15. Accessed 19 October 2021.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

