Lignocellulose molecular assembly and deconstruction properties of lignin-altered rice mutants
- PMID: 36124989
- PMCID: PMC9806629
- DOI: 10.1093/plphys/kiac432
Lignocellulose molecular assembly and deconstruction properties of lignin-altered rice mutants
Abstract
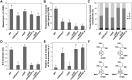
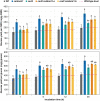
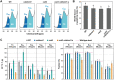
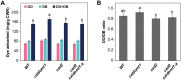
Bioengineering approaches to modify lignin content and structure in plant cell walls have shown promise for facilitating biochemical conversions of lignocellulosic biomass into valuable chemicals. Despite numerous research efforts, however, the effect of altered lignin chemistry on the supramolecular assembly of lignocellulose and consequently its deconstruction in lignin-modified transgenic and mutant plants is not fully understood. In this study, we aimed to close this gap by analyzing lignin-modified rice (Oryza sativa L.) mutants deficient in 5-HYDROXYCONIFERALDEHYDE O-METHYLTRANSFERASE (CAldOMT) and CINNAMYL ALCOHOL DEHYDROGENASE (CAD). A set of rice mutants harboring knockout mutations in either or both OsCAldOMT1 and OsCAD2 was generated in part by genome editing and subjected to comparative cell wall chemical and supramolecular structure analyses. In line with the proposed functions of CAldOMT and CAD in grass lignin biosynthesis, OsCAldOMT1-deficient mutant lines produced altered lignins depleted of syringyl and tricin units and incorporating noncanonical 5-hydroxyguaiacyl units, whereas OsCAD2-deficient mutant lines produced lignins incorporating noncanonical hydroxycinnamaldehyde-derived units. All tested OsCAldOMT1- and OsCAD2-deficient mutants, especially OsCAldOMT1-deficient lines, displayed enhanced cell wall saccharification efficiency. Solid-state nuclear magnetic resonance (NMR) and X-ray diffraction analyses of rice cell walls revealed that both OsCAldOMT1- and OsCAD2 deficiencies contributed to the disruptions of the cellulose crystalline network. Further, OsCAldOMT1 deficiency contributed to the increase of the cellulose molecular mobility more prominently than OsCAD2 deficiency, resulting in apparently more loosened lignocellulose molecular assembly. Such alterations in cell wall chemical and supramolecular structures may in part account for the variations of saccharification performance of the OsCAldOMT1- and OsCAD2-deficient rice mutants.
© American Society of Plant Biologists 2022. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures



References
-
- Abu-Omar MM, Barta K, Beckham GT, Luterbacher JS, Ralph J, Rinaldi R, Román-Leshkov Y, Samec JSM, Sels BF, Wang F (2021) Guidelines for performing lignin-first biorefining. Energy Environ Sci 14: 262–292
-
- Anderson NA, Tobimatsu Y, Ciesielski PN, Ximenes E, Ralph J, Donohoe BS, Ladisch M, Chapple C (2015) Manipulation of guaiacyl and syringyl monomer biosynthesis in an arabidopsis cinnamyl alcohol dehydrogenase mutant results in atypical lignin biosynthesis and modified cell wall structure. Plant Cell 27: 2195–2209 - PMC - PubMed
-
- Biswal AK, Atmodjo MA, Li M, Baxter HL, Yoo CG, Pu Y, Lee YC, Mazarei M, Black IM, Zhang JY, et al. (2018) Sugar release and growth of biofuel crops are improved by downregulation of pectin biosynthesis. Nat Biotechnol 36: 249–257 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

