Cost-effectiveness of direct-acting antivirals for chronic hepatitis C virus in the United States from a payer perspective
- PMID: 36125059
- PMCID: PMC10373042
- DOI: 10.18553/jmcp.2022.28.10.1138
Cost-effectiveness of direct-acting antivirals for chronic hepatitis C virus in the United States from a payer perspective
Abstract
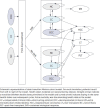
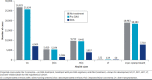
BACKGROUND: Direct-acting antivirals (DAAs) have been a breakthrough therapeutic innovation in the treatment of chronic hepatitis C virus (HCV) with significantly improved efficacy, safety, and tolerability. OBJECTIVE: To evaluate the cost-effectiveness of treating patients with HCV with DAAs compared with pre-DAAs or no treatment over a lifetime horizon from the perspective of the US Veterans Affairs (VA) health care system. METHODS: A hybrid decision-tree and Markov model simulated the health outcomes of a cohort of 142,147 patients with HCV with an average age of 63 years. Demographic data, treatment rates and distribution, treatment efficacy by subpopulation, and health state costs were sourced from VA data. Treatment costs and utility values were sourced from publicly available databases and prior publications for older regimens. RESULTS: Over a lifetime horizon, the use of DAAs results in a significant reduction in advanced liver disease events compared with pre-DAA and no treatment. Total cost savings of $7 and $9 billion over a lifetime horizon (50 years) were predicted for patients who received DAA treatments compared with patients treated with pre-DAA treatments and those who were untreated, respectively. Cost savings were achieved quickly after treatment, with DAAs being inexpensive when compared with both the pre-DAA and untreated scenarios within 5 years. The DAA intervention dominated (ie, more effective and less costly) for both the pre-DAA and untreated strategies on both a per-patient and cohort basis. CONCLUSIONS: The use of DAA-based treatments in patients with HCV in the VA system significantly reduced long-term HCV-related morbidity and mortality, while providing cost savings within only 5 years of treatment. DISCLOSURES: This work was supported by Gilead Inc. Health Economic Outcomes Research group, grant number GS-US-18-HCV003. Drs Yehoshua and Kaushik are employees of Gilead in the Health Economic Outcome Research group. These individuals reviewed the manuscript but did not contribute to input or output of the Markov model. Maple Health Group (Dr El-Moustaid, Ms Raad, and Dr Smith) are consultants hired by Gilead for Markov modeling expertise. The model used in this study was previously published and peer reviewed. Data inputted into the model related to patient demographic, treatment outcomes, clinical outcomes, and costs were completely independent in derivation by Drs Kaplan, Serper, and Durkin and were not influenced by the funding sponsor. Dr Kaplan reports grants from Gilead Inc. during the conduct of the study and grants from Gilead Inc., other from Glycotest Inc., other from AstraZeneca, other from Exact Sciences, and other from Bayer outside the submitted work.
Conflict of interest statement
This work was supported by Gilead Inc. Health Economic Outcomes Research group, grant number GS-US-18-HCV003. Drs Yehoshua and Kaushik are employees of Gilead in the Health Economic Outcome Research group. These individuals reviewed the manuscript but did not contribute to input or output of the Markov model. Maple Health Group (Dr El-Moustaid, Ms Raad, and Dr Smith) are consultants hired by Gilead for Markov modeling expertise. The model used in this study was previously published and peer reviewed. Data inputted into the model related to patient demographic, treatment outcomes, clinical outcomes, and costs were completely independent in derivation by Drs Kaplan, Serper, and Durkin and were not influenced by the funding sponsor. Dr Kaplan reports grants from Gilead Inc. during the conduct of the study and grants from Gilead Inc., other from Glycotest Inc., other from AstraZeneca, other from Exact Sciences, and other from Bayer outside the submitted work.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources