FSH-blocking therapeutic for osteoporosis
- PMID: 36125123
- PMCID: PMC9550223
- DOI: 10.7554/eLife.78022
FSH-blocking therapeutic for osteoporosis
Abstract
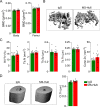
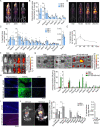
Pharmacological and genetic studies over the past decade have established the follicle-stimulating hormone (FSH) as an actionable target for diseases affecting millions, namely osteoporosis, obesity, and Alzheimer's disease. Blocking FSH action prevents bone loss, fat gain, and neurodegeneration in mice. We recently developed a first-in-class, humanized, epitope-specific FSH-blocking antibody, MS-Hu6, with a KD of 7.52 nM. Using a Good Laboratory Practice (GLP)-compliant platform, we now report the efficacy of MS-Hu6 in preventing and treating osteoporosis in mice and parameters of acute safety in monkeys. Biodistribution studies using 89Zr-labeled, biotinylated or unconjugated MS-Hu6 in mice and monkeys showed localization to bone and bone marrow. The MS-Hu6 displayed a β phase t½ of 7.5 days (180 hr) in humanized Tg32 mice. We tested 217 variations of excipients using the protein thermal shift assay to generate a final formulation that rendered MS-Hu6 stable in solution upon freeze-thaw and at different temperatures, with minimal aggregation, and without self-, cross-, or hydrophobic interactions or appreciable binding to relevant human antigens. The MS-Hu6 showed the same level of "humanness" as human IgG1 in silico and was non-immunogenic in ELISpot assays for IL-2 and IFN-γ in human peripheral blood mononuclear cell cultures. We conclude that MS-Hu6 is efficacious, durable, and manufacturable, and is therefore poised for future human testing.
Keywords: ADME; PK/PD; aging; medicine; menopause; monoclonal antibody; mouse; pituitary hormone; rhesus macaque.
© 2022, Gera, Kuo, Gumerova et al.
Conflict of interest statement
SG, TK, AG, FK, DS, VD, KS, AP, GP, JM, AT, Mv, TP, JF, JN, FS, ES, SR, PK, LC, JC, AP, SM, HK, MB, PS, KI, VM, RB, CR, AM, SH, MS, MM, JC, MN, VR, SK, JC, NZ, ZF, DL, CR No competing interests declared, JI, TY Reviewing editor, eLife, MZ is an inventor on issued patents on inhibiting FSH for the prevention and treatment of osteoporosis and obesity (U.S. Patent 8,435,948 and 11,034,761). M.Z. is also an inventor on pending patent application on composition and use of humanized monoclonal anti-FSH antibodies, and is co-inventor of a pending patent on the use of FSH as a target for preventing Alzheimer's disease. These patents are owned by Icahn School of Medicine at Mount Sinai (ISMMS), and M.Z. would be recipient of royalties, per institutional policy. M.Z. also consults for several financial platforms, including Gerson Lehman Group and Guidepoint, on drugs for osteoporosis and genetic bone diseases. Deputy editor, eLife
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

