Effect of Helmet Noninvasive Ventilation vs Usual Respiratory Support on Mortality Among Patients With Acute Hypoxemic Respiratory Failure Due to COVID-19: The HELMET-COVID Randomized Clinical Trial
- PMID: 36125473
- PMCID: PMC9490511
- DOI: 10.1001/jama.2022.15599
Effect of Helmet Noninvasive Ventilation vs Usual Respiratory Support on Mortality Among Patients With Acute Hypoxemic Respiratory Failure Due to COVID-19: The HELMET-COVID Randomized Clinical Trial
Abstract
Importance: Helmet noninvasive ventilation has been used in patients with COVID-19 with the premise that helmet interface is more effective than mask interface in delivering prolonged treatments with high positive airway pressure, but data about its effectiveness are limited.
Objective: To evaluate whether helmet noninvasive ventilation compared with usual respiratory support reduces mortality in patients with acute hypoxemic respiratory failure due to COVID-19 pneumonia.
Design, setting, and participants: This was a multicenter, pragmatic, randomized clinical trial that was conducted in 8 sites in Saudi Arabia and Kuwait between February 8, 2021, and November 16, 2021. Adult patients with acute hypoxemic respiratory failure (n = 320) due to suspected or confirmed COVID-19 were included. The final follow-up date for the primary outcome was December 14, 2021.
Interventions: Patients were randomized to receive helmet noninvasive ventilation (n = 159) or usual respiratory support (n = 161), which included mask noninvasive ventilation, high-flow nasal oxygen, and standard oxygen.
Main outcomes and measures: The primary outcome was 28-day all-cause mortality. There were 12 prespecified secondary outcomes, including endotracheal intubation, barotrauma, skin pressure injury, and serious adverse events.
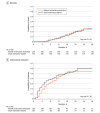
Results: Among 322 patients who were randomized, 320 were included in the primary analysis, all of whom completed the trial. Median age was 58 years, and 187 were men (58.4%). Within 28 days, 43 of 159 patients (27.0%) died in the helmet noninvasive ventilation group compared with 42 of 161 (26.1%) in the usual respiratory support group (risk difference, 1.0% [95% CI, -8.7% to 10.6%]; relative risk, 1.04 [95% CI, 0.72-1.49]; P = .85). Within 28 days, 75 of 159 patients (47.2%) required endotracheal intubation in the helmet noninvasive ventilation group compared with 81 of 161 (50.3%) in the usual respiratory support group (risk difference, -3.1% [95% CI, -14.1% to 7.8%]; relative risk, 0.94 [95% CI, 0.75-1.17]). There were no significant differences between the 2 groups in any of the prespecified secondary end points. Barotrauma occurred in 30 of 159 patients (18.9%) in the helmet noninvasive ventilation group and 25 of 161 (15.5%) in the usual respiratory support group. Skin pressure injury occurred in 5 of 159 patients (3.1%) in the helmet noninvasive ventilation group and 10 of 161 (6.2%) in the usual respiratory support group. There were 2 serious adverse events in the helmet noninvasive ventilation group and 1 in the usual respiratory support group.
Conclusions and relevance: Results of this study suggest that helmet noninvasive ventilation did not significantly reduce 28-day mortality compared with usual respiratory support among patients with acute hypoxemic respiratory failure due to COVID-19 pneumonia. However, interpretation of the findings is limited by imprecision in the effect estimate, which does not exclude potentially clinically important benefit or harm.
Trial registration: ClinicalTrials.gov Identifier: NCT04477668.
Conflict of interest statement
Figures

References
-
- Perkins GD, Ji C, Connolly BA, et al. ; RECOVERY-RS Collaborators . Effect of noninvasive respiratory strategies on intubation or mortality among patients with acute hypoxemic respiratory failure and COVID-19: the RECOVERY-RS randomized clinical trial. JAMA. 2022;327(6):546-558. doi:10.1001/jama.2022.0028 - DOI - PMC - PubMed
-
- Reyes LF, Murthy S, Garcia-Gallo E, et al. ; ISARIC Clinical Characterisation Group . Clinical characteristics, risk factors and outcomes in patients with severe COVID-19 registered in the International Severe Acute Respiratory and Emerging Infection Consortium WHO clinical characterisation protocol: a prospective, multinational, multicentre, observational study. ERJ Open Res. 2022;8(1):00552-2021. doi:10.1183/23120541.00552-2021 - DOI - PMC - PubMed

