A member of the tryptophan-rich protein family is required for efficient sequestration of Plasmodium berghei schizonts
- PMID: 36126089
- PMCID: PMC9524624
- DOI: 10.1371/journal.ppat.1010846
A member of the tryptophan-rich protein family is required for efficient sequestration of Plasmodium berghei schizonts
Abstract
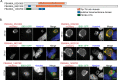
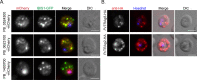
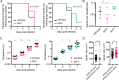
Protein export and host membrane remodeling are crucial for multiple Plasmodium species to establish a niche in infected hosts. To better understand the contribution of these processes to successful parasite infection in vivo, we sought to find and characterize protein components of the intraerythrocytic Plasmodium berghei-induced membrane structures (IBIS) that form in the cytoplasm of infected erythrocytes. We identified proteins that immunoprecipitate with IBIS1, a signature member of the IBIS in P. berghei-infected erythrocytes. In parallel, we also report our data describing proteins that co-precipitate with the PTEX (Plasmodium translocon of exported proteins) component EXP2. To validate our findings, we examined the location of three candidate IBIS1-interactors that are conserved across multiple Plasmodium species, and we found they localized to IBIS in infected red blood cells and two further colocalized with IBIS1 in the liver-stage parasitophorous vacuole membrane. Successful gene deletion revealed that these two tryptophan-rich domain-containing proteins, termed here IPIS2 and IPIS3 (for intraerythrocytic Plasmodium-induced membrane structures), are required for efficient blood-stage growth. Erythrocytes infected with IPIS2-deficient schizonts in particular fail to bind CD36 as efficiently as wild-type P. berghei-infected cells and therefore fail to effectively sequester out of the circulating blood. Our findings support the idea that intra-erythrocytic membrane compartments are required across species for alterations of the host erythrocyte that facilitate interactions of infected cells with host tissues.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

