Control of myopia using diffusion optics spectacle lenses: 12-month results of a randomised controlled, efficacy and safety study (CYPRESS)
- PMID: 36126105
- PMCID: PMC10646852
- DOI: 10.1136/bjo-2021-321005
Control of myopia using diffusion optics spectacle lenses: 12-month results of a randomised controlled, efficacy and safety study (CYPRESS)
Abstract
Background: Mutations in the L/M cone opsin gene array cause abnormally high perceived retinal contrast and the development of myopia. Environmental factors may also lead to high visual contrast and cause myopia. Diffusion optics technology (DOT) lenses are designed to reduce contrast signalling in the retina and slow myopia progression.
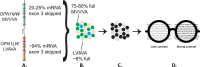
Methods: The Control of Myopia Using Peripheral Diffusion Lenses Efficacy and Safety Study (CYPRESS, NCT03623074) is a 36-month, multicentre, randomised, controlled, double-masked trial evaluating two investigational spectacle lenses versus control lenses in myopic children aged 6-10, with a planned interim analysis at 12 months. The primary endpoints are change from baseline in axial length (AL) and spherical equivalent refraction (SER).
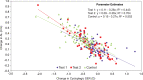
Results: 256 children (58% female; mean age at screening, 8.1 years) were dispensed spectacles. Across all groups, baseline averages were AL 24.02 mm (SD±0.77 mm), SER -2.01 D (SD±0.9 D) using manifest refraction, and SER -1.94 D (SD±1.0 D) using cycloplegic autorefraction. At 12 months, mean difference in SER progression for test 1 versus control was -0.40 D (p<0.0001), representing a 74% reduction and -0.32 D for Test 2 (p<0.0001), representing a 59% reduction. The difference in AL progression for test 1 versus control was 0.15 mm (p<0.0001) and test 2 versus control was 0.10 mm (p=0.0018).
Conclusion: 12-month results from this ongoing trial demonstrate the safety and effectiveness of DOT spectacles for reducing myopic progression.
Keywords: Clinical Trial; Treatment other; Vision.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: JR is former Chief Medical Officer at SightGlass Vision, and in this role has received meeting and/or travel support, participated in data safety monitoring/advisory boards, and owns stock in the company. CC reports consulting fees from SightGlass Vision and has a pending patent application (17/259,779). GY reports grants from Johnson & Johnson Vision, CooperVision, and Essilor. JN and MN report royalties/licenses, consulting fees and meeting and/or travel support from SightGlass Vision. JN and MN are cofounders and have stock ownership in SightGlass Vision, and are listed as Inventors on patents issued for DOT lens, owned by the University of Washington. TC reports consulting fees, meeting and/or travel support, patents and stock ownership in his role as employee, officer and board member for SightGlass Vision.
Figures

References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
