When laughter arrests speech: fMRI-based evidence
- PMID: 36126674
- PMCID: PMC9489293
- DOI: 10.1098/rstb.2021.0182
When laughter arrests speech: fMRI-based evidence
Abstract
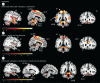
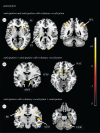
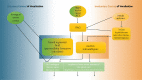
Who has not experienced that sensation of losing the power of speech owing to an involuntary bout of laughter? An investigation of this phenomenon affords an insight into the neuronal processes that underlie laughter. In our functional magnetic resonance imaging study, participants were made to laugh by tickling in a first condition; in a second one they were requested to produce vocal utterances under the provocation of laughter by tickling. This investigation reveals increased neuronal activity in the sensorimotor cortex, the anterior cingulate gyrus, the insula, the nucleus accumbens, the hypothalamus and the periaqueductal grey for both conditions, thereby replicating the results of previous studies on ticklish laughter. However, further analysis indicates the activity in the emotion-associated regions to be lower when tickling is accompanied by voluntary vocalization. Here, a typical pattern of activation is identified, including the primary sensory cortex, a ventral area of the anterior insula and the ventral tegmental field, to which belongs to the nucleus ambiguus, namely, the common effector organ for voluntary and involuntary vocalizations. During the conflictual voluntary-vocalization versus laughter experience, the laughter-triggering network appears to rely heavily on a sensory and a deep interoceptive analysis, as well as on motor effectors in the brainstem. This article is part of the theme issue 'Cracking the laugh code: laughter through the lens of biology, psychology and neuroscience'.
Keywords: fMRI; laughter; tickle; touch; vocalization.
Figures
References
-
- Roccaro-Waldmeyer DM, Babalian A, Muller A, Celio MR. 2016. Reduction in 50-kHz call-numbers and suppression of tickling-associated positive affective behaviour after lesioning of the lateral hypothalamic parvafox nucleus in rats. Behav. Brain Res. 298, 167-180. (10.1016/j.bbr.2015.11.004) - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
