Antisense pairing and SNORD13 structure guide RNA cytidine acetylation
- PMID: 36127124
- PMCID: PMC9670809
- DOI: 10.1261/rna.079254.122
Antisense pairing and SNORD13 structure guide RNA cytidine acetylation
Abstract
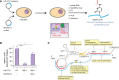
N4-acetylcytidine (ac4C) is an RNA nucleobase found in all domains of life. The establishment of ac4C in helix 45 (h45) of human 18S ribosomal RNA (rRNA) requires the combined activity of the acetyltransferase NAT10 and the box C/D snoRNA SNORD13. However, the molecular mechanisms governing RNA-guided nucleobase acetylation in humans remain unexplored. After applying comparative sequence analysis and site-directed mutagenesis to provide evidence that SNORD13 folds into three main RNA helices, we report two assays that enable the study of SNORD13-dependent RNA acetylation in human cells. First, we demonstrate that ectopic expression of SNORD13 rescues h45 in a SNORD13 knockout cell line. Next, we show that mutant snoRNAs can be used in combination with nucleotide resolution ac4C sequencing to define structure and sequence elements critical for SNORD13 function. Finally, we develop a second method that reports on the substrate specificity of endogenous NAT10-SNORD13 via mutational analysis of an ectopically expressed pre-rRNA substrate. By combining mutational analysis of these reconstituted systems with nucleotide resolution ac4C sequencing, our studies reveal plasticity in the molecular determinants underlying RNA-guided cytidine acetylation that is distinct from deposition of other well-studied rRNA modifications (e.g., pseudouridine). Overall, our studies provide a new approach to reconstitute RNA-guided cytidine acetylation in human cells as well as nucleotide resolution insights into the mechanisms governing this process.
Keywords: N4-acetylcytidine; SNORD13; epitranscriptome; modification; ribosome.
© 2022 Thalalla Gamage et al.; Published by Cold Spring Harbor Laboratory Press for the RNA Society.
Figures






References
-
- Badrock AP, Uggenti C, Wacheul L, Crilly S, Jenkinson EM, Rice GI, Kasher PR, Lafontaine DLJ, Crow YJ, O'Keefe RT. 2020. Analysis of U8 snoRNA variants in zebrafish reveals how bi-allelic variants cause leukoencephalopathy with calcifications and cysts. Am J Hum Genet 106: 694–706. 10.1016/j.ajhg.2020.04.003 - DOI - PMC - PubMed
-
- Badrock AP, Uggenti C, Wachul L, Crilly S, Jenkinson EM, Rice GI, Kasher PR, Lafontaine DL, Crow Y, O'Keefe RT. 2022. Novel intramolecular base-pairing of the U8 snoRNA underlies a Mendelian form of cerebral small vessel disease. bioRxiv 10.1101/2019.12.12.874594 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
