Enhanced oxygen evolution over dual corner-shared cobalt tetrahedra
- PMID: 36127321
- PMCID: PMC9489709
- DOI: 10.1038/s41467-022-33000-w
Enhanced oxygen evolution over dual corner-shared cobalt tetrahedra
Abstract
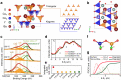
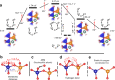
Developing efficient catalysts is of paramount importance to oxygen evolution, a sluggish anodic reaction that provides essential electrons and protons for various electrochemical processes, such as hydrogen generation. Here, we report that the oxygen evolution reaction (OER) can be efficiently catalyzed by cobalt tetrahedra, which are stabilized over the surface of a Swedenborgite-type YBCo4O7 material. We reveal that the surface of YBaCo4O7 possesses strong resilience towards structural amorphization during OER, which originates from its distinctive structural evolution toward electrochemical oxidation. The bulk of YBaCo4O7 composes of corner-sharing only CoO4 tetrahedra, which can flexibly alter their positions to accommodate the insertion of interstitial oxygen ions and mediate the stress during the electrochemical oxidation. The density functional theory calculations demonstrate that the OER is efficiently catalyzed by a binuclear active site of dual corner-shared cobalt tetrahedra, which have a coordination number switching between 3 and 4 during the reaction. We expect that the reported active structural motif of dual corner-shared cobalt tetrahedra in this study could enable further development of compounds for catalyzing the OER.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures