Current understanding of osteoarthritis pathogenesis and relevant new approaches
- PMID: 36127328
- PMCID: PMC9489702
- DOI: 10.1038/s41413-022-00226-9
Current understanding of osteoarthritis pathogenesis and relevant new approaches
Abstract
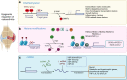
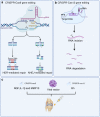
Osteoarthritis (OA) is the most common degenerative joint disease that causes painful swelling and permanent damage to the joints in the body. The molecular mechanisms of OA are currently unknown. OA is a heterogeneous disease that affects the entire joint, and multiple tissues are altered during OA development. To better understand the pathological mechanisms of OA, new approaches, methods, and techniques need to be used to understand OA pathogenesis. In this review, we first focus on the epigenetic regulation of OA, with a particular focus on DNA methylation, histone modification, and microRNA regulation, followed by a summary of several key mediators in OA-associated pain. We then introduce several innovative techniques that have been and will continue to be used in the fields of OA and OA-associated pain, such as CRISPR, scRNA sequencing, and lineage tracing. Next, we discuss the timely updates concerning cell death regulation in OA pathology, including pyroptosis, ferroptosis, and autophagy, as well as their individual roles in OA and potential molecular targets in treating OA. Finally, our review highlights new directions on the role of the synovial lymphatic system in OA. An improved understanding of OA pathogenesis will aid in the development of more specific and effective therapeutic interventions for OA.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
Grants and funding
- 82161160342/National Natural Science Foundation of China (National Science Foundation of China)
- 82172397/National Natural Science Foundation of China (National Science Foundation of China)
- 2020353/Youth Innovation Promotion Association of the Chinese Academy of Sciences (Youth Innovation Promotion Association CAS)
LinkOut - more resources
Full Text Sources

