KSHV (HHV8) vaccine: promises and potential pitfalls for a new anti-cancer vaccine
- PMID: 36127367
- PMCID: PMC9488886
- DOI: 10.1038/s41541-022-00535-4
KSHV (HHV8) vaccine: promises and potential pitfalls for a new anti-cancer vaccine
Abstract
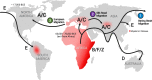
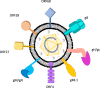
Seven viruses cause at least 15% of the total cancer burden. Viral cancers have been described as the "low-hanging fruit" that can be potentially prevented or treated by new vaccines that would alter the course of global human cancer. Kaposi sarcoma herpesvirus (KSHV or HHV8) is the sole cause of Kaposi sarcoma, which primarily afflicts resource-poor and socially marginalized populations. This review summarizes a recent NIH-sponsored workshop's findings on the epidemiology and biology of KSHV as an overlooked but potentially vaccine-preventable infection. The unique epidemiology of this virus provides opportunities to prevent its cancers if an effective, inexpensive, and well-tolerated vaccine can be developed and delivered.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests. P.S.M. has a KSHV vaccine patent assigned to the University of Pittsburgh, J.G.O. has a KSHV vaccine patent assigned to the Beckman Research Institute of City of and L.T.K. has a patent for generating reversion-free attenuated and/or replication-incompetent vaccine vectors and has divested herself of potential earnings.
Figures

References
-
- Chang, Y., Gao, S.-J. & Moore, P. S. In Clinical Virology (eds Richman, D. D., Whitley, R. J., Hayden, F. G.) 549–574 (ASM Press, 2016).

