Solar-light-driven LaFe x Ni1- x O3 perovskite oxides for photocatalytic Fenton-like reaction to degrade organic pollutants
- PMID: 36127897
- PMCID: PMC9475182
- DOI: 10.3762/bjnano.13.79
Solar-light-driven LaFe x Ni1- x O3 perovskite oxides for photocatalytic Fenton-like reaction to degrade organic pollutants
Abstract
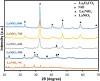
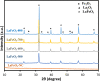
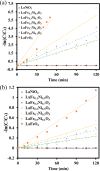
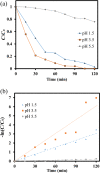
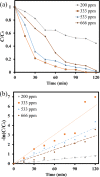
LaFe x Ni1- x O3 perovskite oxides were prepared by the sol-gel method under various conditions, including different pH values (pH 0 and pH 7) and different calcination temperatures (500-800 °C) as well as different Fe/Ni ratios (1/9, 3/7, 5/5, 7/3, 9/1). The samples were examined by XRD, DRS, BET, and SEM to reveal their crystallinity, light-absorption ability, specific surface area, and surface features, respectively. The photocatalytic Fenton reaction was conducted using various LaFe x Ni1- x O3 perovskite oxides to decompose the methylene blue molecules. Accordingly, the synthesis condition of pH 0, calcination temperature at 700 °C, and Fe/Ni ratio = 7/3 could form LaFe0.7Ni0.3O3 perovskite oxides as highly efficient photocatalysts. Moreover, various conditions during the photocatalytic degradation were verified, such as pH value, catalyst dosage, and the additional amount of H2O2. LaFe0.7Ni0.3O3 perovskite oxides could operate efficiently under pH 3.5, catalyst dosage of 50 mg/150 mL, and H2O2 concentration of 133 ppm to decompose the MB dye in the 1st order kinetic rate constant of 0.0506 s-1.
Keywords: LaFeO3; LaNiO3; methylene blue (MB); perovskite oxides; photocatalyst.
Copyright © 2022, Huang et al.
Figures







Similar articles
-
Synthesis, characterization, and visible-light-induced photocatalytic activity of powdered semiconductor oxides of bismuth and zinc toward degradation of Alizarin Red S.Water Environ Res. 2020 Sep;92(9):1376-1387. doi: 10.1002/wer.1333. Epub 2020 May 1. Water Environ Res. 2020. PMID: 32221996
-
Effect of calcination temperature, pH and catalyst loading on photodegradation efficiency of urea derived graphitic carbon nitride towards methylene blue dye solution.RSC Adv. 2019 May 16;9(27):15381-15391. doi: 10.1039/c9ra02201e. eCollection 2019 May 14. RSC Adv. 2019. PMID: 35514817 Free PMC article.
-
Preparation of new adsorbent-supported Fe/Ni particles for the removal of crystal violet and methylene blue by a heterogeneous Fenton-like reaction.RSC Adv. 2019 Jul 22;9(39):22513-22522. doi: 10.1039/c9ra04710g. eCollection 2019 Jul 17. RSC Adv. 2019. PMID: 35519486 Free PMC article.
-
Cationic and anionic cross-assisted synergistic photocatalytic removal of binary organic dye mixture using Ni-doped perovskite oxide.Chemosphere. 2023 Nov;340:139890. doi: 10.1016/j.chemosphere.2023.139890. Epub 2023 Aug 22. Chemosphere. 2023. PMID: 37619747
-
Preparation of Cotton Linters' Aerogel-Based C/NiFe2O4 Photocatalyst for Efficient Degradation of Methylene Blue.Nanomaterials (Basel). 2022 Jun 11;12(12):2021. doi: 10.3390/nano12122021. Nanomaterials (Basel). 2022. PMID: 35745360 Free PMC article.
References
-
- Otto B, Schleifer L. Domestic Water Use Grew 600% Over the Past 50 Years. World Resources Institute; 2020. [ Aug 15; 2022 ]. Available from: https://www.wri.org/insights/domestic-water-use-grew-600-over-past-50-years.
-
- Searchinger T, Waite R, Hanson C, et al. Creating a Sustainable Food Future. World Resources Institute; 2018. - DOI
-
- Lin Y-T, Wang Y-H, Wu J C S, Wang X. Appl Catal, B. 2021;281:119517. doi: 10.1016/j.apcatb.2020.119517. - DOI
-
- Lin Y-T, Huang C-W, Wang Y-H, Wu J C S. Top Catal. 2020;63(11-14):1240–1250. doi: 10.1007/s11244-020-01263-6. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
