Ad26.COV2.S Vaccine-Induced Thrombocytopenia Leading to Dural Sinus Thrombosis and Intracranial Hemorrhage Requiring Hemicraniectomy: A Case Report and Systematic Review
- PMID: 36127984
- PMCID: PMC9477649
- DOI: 10.7759/cureus.28083
Ad26.COV2.S Vaccine-Induced Thrombocytopenia Leading to Dural Sinus Thrombosis and Intracranial Hemorrhage Requiring Hemicraniectomy: A Case Report and Systematic Review
Abstract
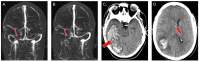
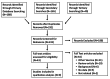
The coronavirus disease 2019 (COVID-19) pandemic has claimed nearly 5.5 million lives worldwide. Adenovirus-based vaccines are safe and effective, but they are rarely associated with vaccine-induced thrombosis and thrombocytopenia (VITT) as well as cerebral venous sinus thrombosis (CVST). We conducted a systematic literature search of intracerebral hemorrhage (ICH) secondary to CVST associated with VITT from the Ad26.COV2.S vaccine, and we present the first case of this pathology in the reviewed literature of a patient who required neurosurgical decompression. The systematic literature review was completed on December 19, 2021, by searching PubMed and Ovid for articles with primary data on CVST associated with VITT following the Ad26.COV2.S vaccine. We also specifically searched for cases that required neurosurgical intervention. Articles were independently screened by two authors, and both secondary and tertiary searches were done as well. Descriptive statistics were collected and presented in table form. Nine studies were identified that met inclusion criteria. There were no cases identified of patients who underwent neurosurgical decompression after developing this pathology. We thus present the first case in the reviewed literature of a patient who developed ICH after receiving the Ad26.COV2.S vaccine and underwent decompressive hemicraniectomy. Despite severe thrombocytopenia and prolonged intensive care, the patient was discharged to neurorehabilitation. There is a much greater risk of CVST and ICH during COVID-19 infections than from the vaccines. However, as booster vaccines are approved and widely distributed, it is critical to make prompt, accurate diagnoses of this vaccine-related complication and consider neurosurgical decompression.
Keywords: ad26-cov2-s; cerebral venous sinus thrombosis (cvst); covid-19; craniectomy; intracranial hemorrhage; neurosurgical decompression; vaccine-induced thrombosis and thrombocytopenia (vitt).
Copyright © 2022, Daly et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
US Case Reports of Cerebral Venous Sinus Thrombosis With Thrombocytopenia After Ad26.COV2.S Vaccination, March 2 to April 21, 2021.JAMA. 2021 Jun 22;325(24):2448-2456. doi: 10.1001/jama.2021.7517. JAMA. 2021. PMID: 33929487 Free PMC article.
-
Vaccine-induced immune thrombotic thrombocytopenia in a male after Ad26.COV2.S vaccination presenting as cerebral venous sinus thrombosis.Platelets. 2022 Jul 4;33(5):797-800. doi: 10.1080/09537104.2022.2071854. Epub 2022 May 9. Platelets. 2022. PMID: 35535430
-
Comparison of vaccine-induced immune thrombocytopenia and thrombosis cases following two adenovirus-vectored COVID-19 vaccines.Commun Med (Lond). 2025 May 10;5(1):168. doi: 10.1038/s43856-025-00891-x. Commun Med (Lond). 2025. PMID: 40348868 Free PMC article.
-
Ischemic Stroke and Vaccine-Induced Immune Thrombotic Thrombocytopenia following COVID-19 Vaccine: A Case Report with Systematic Review of the Literature.Cerebrovasc Dis. 2022;51(6):722-734. doi: 10.1159/000524290. Epub 2022 May 5. Cerebrovasc Dis. 2022. PMID: 35512656 Free PMC article.
-
Thrombosis with Thrombocytopenia Syndrome After Administration of AZD1222 or Ad26.COV2.S Vaccine for COVID-19: A Systematic Review.Clin Appl Thromb Hemost. 2021 Jan-Dec;27:10760296211068487. doi: 10.1177/10760296211068487. Clin Appl Thromb Hemost. 2021. PMID: 34907794 Free PMC article.
Cited by
-
Construction and application of adenoviral vectors.Mol Ther Nucleic Acids. 2023 Sep 9;34:102027. doi: 10.1016/j.omtn.2023.09.004. eCollection 2023 Dec 12. Mol Ther Nucleic Acids. 2023. PMID: 37808925 Free PMC article. Review.
References
-
- Coronavirus disease (COVID-19) pandemic. [ Oct; 2021 ]. 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019 https://www.who.int/emergencies/diseases/novel-coronavirus-2019
-
- Cerebral venous thrombosis. Ropper AH, Klein JP. N Engl J Med. 2021;385:59–64. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
