Room temperature charge-transfer phosphorescence from organic donor-acceptor Co-crystals
- PMID: 36128227
- PMCID: PMC9430718
- DOI: 10.1039/d2sc03343g
Room temperature charge-transfer phosphorescence from organic donor-acceptor Co-crystals
Abstract
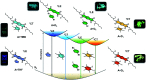
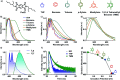
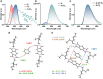
Engineering the electronic excited state manifolds of organic molecules can give rise to various functional outcomes, including ambient triplet harvesting, that has received prodigious attention in the recent past. Herein, we introduce a modular, non-covalent approach to bias the entire excited state landscape of an organic molecule using tunable 'through-space charge-transfer' interactions with appropriate donors. Although charge-transfer (CT) donor-acceptor complexes have been extensively explored as functional and supramolecular motifs in the realm of soft organic materials, they could not imprint their potentiality in the field of luminescent materials, and it still remains as a challenge. Thus, in the present study, we investigate the modulation of the excited state emission characteristics of a simple pyromellitic diimide derivative on complexation with appropriate donor molecules of varying electronic characteristics to demonstrate the selective harvesting of emission from its locally excited (LE) and CT singlet and triplet states. Remarkably, co-crystallization of the pyromellitic diimide with heavy-atom substituted and electron-rich aromatic donors leads to an unprecedented ambient CT phosphorescence with impressive efficiency and notable lifetime. Further, gradual minimizing of the electron-donating strength of the donors from 1,4-diiodo-2,3,5,6-tetramethylbenzene (or 1,2-diiodo-3,4,5,6-tetramethylbenzene) to 1,2-diiodo-4,5-dimethylbenzene and 1-bromo-4-iodobenzene modulates the source of ambient phosphorescence emission from the 3CT excited state to 3LE excited state. Through comprehensive spectroscopic, theoretical studies, and single-crystal analyses, we elucidate the unparalleled role of intermolecular donor-acceptor interactions to toggle between the emissive excited states and stabilize the triplet excitons. We envisage that the present study will be able to provide new and innovative dimensions to the existing molecular designs employed for triplet harvesting.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


References
-
- Hirata S. Adv. Opt. Mater. 2017;5:1700116. doi: 10.1002/adom.201700116. - DOI
- Wong M. Y. Zysman-Colman E. Adv. Mater. 2017;29:1605444. doi: 10.1002/adma.201605444. - DOI - PubMed
- Zhao W. He Z. Jacky W. Y. L. Peng Q. Ma H. Shuai Z. Bai G. Hao J. Tang B. Z. Chem. 2016;1:592–602. doi: 10.1016/j.chempr.2016.08.010. - DOI
- Xu S. Chen R. Zheng C. Huang W. Adv. Mater. 2016;28:9920–9940. doi: 10.1002/adma.201602604. - DOI - PubMed
- Zhao W. He Z. Tang B. Z. Nat. Rev. Mater. 2020;5:869–885. doi: 10.1038/s41578-020-0223-z. - DOI
- Ma X. Wang J. Tian H. Acc. Chem. Res. 2019;52:738–748. doi: 10.1021/acs.accounts.8b00620. - DOI - PubMed
- Zhang T. Ma X. Wu H. Zhu L. Zhao Y. Tian H. Angew. Chem., Int. Ed. 2020;59:11206–11216. doi: 10.1002/anie.201915433. - DOI - PubMed
- Wang Y. Gao H. Yang J. Fang M. Ding D. Tang B. Li Z. Adv. Mater. 2021;33:2007811. doi: 10.1002/adma.202007811. - DOI - PubMed
- Nidhankar A. D. Goudappagouda Wakchaure V. C. Babu S. S. Chem. Sci. 2021;12:4216–4236. doi: 10.1039/D1SC00446H. - DOI - PMC - PubMed
- Hackney H. E. Perepichka D. F. Aggregate. 2021:e123.
- Gao H. Ma X. Aggregate. 2021;2:e38.
-
- Bolton O. Lee K. Kim J.-H. Lin K. Y. Kim J. Nat. Chem. 2011;3:205–210. doi: 10.1038/nchem.984. - DOI - PubMed
- An Z. Zheng C. Tao Y. Chen R. Shi H. Chen T. Wang Z. Li H. Deng R. Li X. Huang W. Nat. Mater. 2015;14:685–690. doi: 10.1038/nmat4259. - DOI - PubMed
- Cai S. Shi H. Li J. Gu L. Cheng Y. N. Z. Wang S. Xiong W. Li L. An Z. Huang W. Adv. Mater. 2017;29:1701244. doi: 10.1002/adma.201701244. - DOI - PubMed
- Alam P. Cheung T. S. Leung N. L. C. Zhang J. Guo J. Du L. Kwok R. T. K. Lam J. W. Y. Zeng Z. Phillips D. L. Sung H. H. Y. Williams I. D. Tang B. Z. J. Am. Chem. Soc. 2022;144:3050–3062. doi: 10.1021/jacs.1c11480. - DOI - PubMed
- Gong Y. Zhao L. Peng Q. Fan D. Yuan W. Zhang Y. Tang B. Z. Chem. Sci. 2015;6:4438–4444. doi: 10.1039/C5SC00253B. - DOI - PMC - PubMed
- Alam P. Leung N. Liu J. Zhang X. He Z. Kwok R. T. K. Lam J. W. Y. Sung H. H. Y. Williams I. D. Peng Q. Tang B. Z. Adv. Mater. 2020;32:2001026. doi: 10.1002/adma.202001026. - DOI - PubMed
- Wang Y. Yang J. Tian Y. Fang M. Liao Q. Wang L. Hu W. Tang B. Z. Li Z. Chem. Sci. 2020;11:833–838. doi: 10.1039/C9SC04632A. - DOI - PMC - PubMed
- Liao Q. Gao Q. Wang J. Gong Y. Peng Q. Tian Y. Fan Y. Guo H. Ding D. Li Q. Li Z. Angew. Chem., Int. Ed. 2020;59:9946–9951. doi: 10.1002/anie.201916057. - DOI - PubMed
- Ren J. Wang Y. Tian Y. Liu Z. Xiao X. Yang J. Fang M. Li Z. Angew. Chem., Int. Ed. 2021;60:12335–12340. doi: 10.1002/anie.202101994. - DOI - PubMed
- Zhang Z.-Y. Liu Y. Chem. Sci. 2019;10:7773–7778. doi: 10.1039/C9SC02633A. - DOI - PMC - PubMed
-
- Kwon M. S. Lee D. Seo S. Jung J. Kim J. Angew. Chem., Int. Ed. 2014;53:11177–11181. doi: 10.1002/anie.201404490. - DOI - PubMed
- Kuila S. Garain S. Bandi S. George S. J. Adv. Funct. Mater. 2020;30:2003693. doi: 10.1002/adfm.202003693. - DOI
- Wu H. Chi W. Chen Z. Liu G. Gu L. Bindra A. K. Yang G. Liu X. Zhao Y. Adv. Funct. Mater. 2019;29:1807243. doi: 10.1002/adfm.201807243. - DOI
- Wang Z. Zhang Y. Wang C. Zheng X. Zheng Y. Gao L. Yang C. Li Y. Qu L. Zhao Y. Adv. Mater. 2020;32:1907355. doi: 10.1002/adma.201907355. - DOI - PubMed
- Su Y. Zhang Y. Wang Z. Gao W. Jia P. Zhang D. Yang C. Li Y. Zhao Y. Angew. Chem., Int. Ed. 2020;59:9967–9971. doi: 10.1002/anie.201912102. - DOI - PubMed
- Wang C. Zhang Y. Wang Z. Zheng Y. Zheng X. Gao L. Zhou Q. Hao J. Pi B. Li Q. Yang C. Li Y. Wang K. Zhao Y. Adv. Funct. Mater. 2022;32:2111941. doi: 10.1002/adfm.202111941. - DOI
- Cai S. Sun Z. Wang H. Yao X. Ma H. Jia W. Wang S. Li Z. Shi H. An Z. Ishida Y. Aida T. Huang W. J. Am. Chem. Soc. 2021;143:16256–16263. doi: 10.1021/jacs.1c07674. - DOI - PubMed
- Louis M. Thomas H. Gmelch M. Haft A. Fries F. Reineke S. Adv. Mater. 2019;31:1807887. doi: 10.1002/adma.201807887. - DOI - PubMed
- Thomas H. Pastoetter D. L. Gmelch M. Achenbach T. Schlçgl A. Louis M. Feng X. Reineke S. Adv. Mater. 2020;32:2000880. doi: 10.1002/adma.202000880. - DOI - PubMed
-
- Wang J. Li Z. D. Lu F. Wang J. Hu W. Cao X. M. Ma X. Tian H. J. Am. Chem. Soc. 2018;140:1916–1923. doi: 10.1021/jacs.7b12800. - DOI - PubMed
- Wang J. Huang Z. Ma X. Tian H. Angew. Chem., Int. Ed. 2020;59:9928–9933. doi: 10.1002/anie.201914513. - DOI - PubMed
- Yao X. Wang J. Jiao D. Huang Z. Mhirsi O. Lossada F. Chen L. Haehnle B. Kuehne A. J. C. Ma X. Tian H. Walther A. Adv. Mater. 2020:2005973. - PMC - PubMed
- Zhang Z. Y. Chen Y. Liu Y. Angew. Chem., Int. Ed. 2019;58:6028–6032. doi: 10.1002/anie.201901882. - DOI - PubMed
- Huo M. Dai X. Y. Liu Y. Angew. Chem., Int. Ed. 2021;60:27171–27177. doi: 10.1002/anie.202113577. - DOI - PubMed
- Zhang Z.-Y. Xu W.-W. Xu W.-S. Niu J. Sun X.-H. Liu Y. Angew. Chem., Int. Ed. 2020;59:18748–18754. doi: 10.1002/anie.202008516. - DOI - PubMed
- Sun S. Wang J. Ma L. Ma X. Tian H. Angew. Chem., Int. Ed. 2021;60:18557–18560. doi: 10.1002/anie.202107323. - DOI - PubMed
- Shen F.-F. Chen Y. Dai X. Zhang H.-Y. Zhang B. Liu Y.-H. Liu Y. Chem. Sci. 2021;12:1851–1857. doi: 10.1039/D0SC05343K. - DOI - PMC - PubMed
- Zhou W. L. Lin W. Chen Y. Dai X. Y. Liu Z. Liu Y. Chem. Sci. 2022;13:573–579. doi: 10.1039/D1SC05861D. - DOI - PMC - PubMed
- Li Z. Han Y. Nie F. Liu M. Zhong H. Wang F. Angew. Chem., Int. Ed. 2021;60:8212–8219. doi: 10.1002/anie.202015846. - DOI - PubMed
- Li Z. Han Y. Wang F. Nat. Commun. 2019;10:3735. doi: 10.1038/s41467-019-11650-7. - DOI - PMC - PubMed
- Kuila S. Rao K. V. Garain S. Samanta P. K. Das S. Pati S. K. Eswaramoorthy M. George S. J. Angew. Chem., Int. Ed. 2018;57:17115–17119. doi: 10.1002/anie.201810823. - DOI - PubMed
- Garain S. Garain B. C. Eswaramoorthy M. Pati S. K. George S. J. Angew. Chem., Int. Ed. 2021;60:19720–19724. doi: 10.1002/anie.202107295. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

